Novel Liposomal Rolipram Formulation for Clinical Application to Reduce Emesis
- PMID: 35535222
- PMCID: PMC9078351
- DOI: 10.2147/DDDT.S355796
Novel Liposomal Rolipram Formulation for Clinical Application to Reduce Emesis
Abstract
Introduction: The phosphodiesterase 4 (PDE4) inhibitor, rolipram, has beneficial effects on tissue inflammation, injury and fibrosis, including in the liver. Since rolipram elicits significant CNS side-effects in humans (ie, nausea and emesis), our group developed a fusogenic lipid vesicle (FLV) drug delivery system that targets the liver to avoid adverse events. We evaluated whether this novel liposomal rolipram formulation reduces emesis.
Methods: C57Bl/6J male mice were used to compare the effect of three doses of free and FLV-delivered (FLVs-Rol) rolipram in a behavioral correlate model of rolipram-induced emesis. Tissue rolipram and rolipram metabolite levels were measured using LC-MS/MS. The effect of FLVs-Rol on brain and liver PDE4 activities was evaluated.
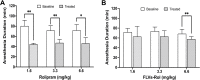
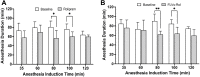
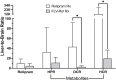
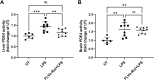
Results: Low and moderate doses of free rolipram significantly reduced anesthesia duration, while the same doses of FLVs-Rol had no effect. However, the onset and duration of adverse effects (shortening of anesthesia period) elicited by a high dose of rolipram was not ameliorated by FLVs-Rol. Post-mortem analysis of brain and liver tissues demonstrated that FLVs affected the rate of rolipram uptake by liver and brain. Lastly, administration of a moderate dose of FLVs-Rol attenuated endotoxin induced PDE4 activity in the liver with negligible effect on the brain.
Discussion: The findings that the low and moderate doses of FLVs-Rol did not shorten the anesthesia duration time suggest that FLV delivery prevented critical levels of drug from crossing the blood-brain barrier (BBB) to elicit CNS side-effects. However, the inability of high dose FLVs-Rol to prevent CNS side-effects indicates that there was sufficient unencapsulated rolipram to cross the BBB and shorten anesthesia duration. Notably, a moderate dose of FLVs-Rol was able to decrease PDE4 activity in the liver without affecting the brain. Taken together, FLVs-Rol has a strong potential for clinical application for the treatment of liver disease without side effects.
Keywords: PDE4; liposomes; liver; rolipram; side-effects.
© 2022 Gobejishvili et al.
Conflict of interest statement
Drs. Maldonado and Bauer declare a conflict of interest in employment and having equity in EndoProtech, Inc. The authors report no other conflicts of interest in this work.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

