Evaluation of conventional and point-of-care real-time RT-PCR tests for the detection of SARS-CoV-2 through a pooled testing strategy
- PMID: 35535393
- PMCID: PMC9169176
- DOI: 10.1002/jcla.24491
Evaluation of conventional and point-of-care real-time RT-PCR tests for the detection of SARS-CoV-2 through a pooled testing strategy
Abstract
Background: The rapid identification and isolation of individuals infected with SARS-CoV-2 are fundamental countermeasures for the efficient control of the COVID-19 pandemic, which has affected millions of people around the world. Real-time RT-PCR is one of the most commonly applied reference methods for virus detection, and the use of pooled testing has been proposed as an effective way to increase the throughput of routine diagnostic tests. However, the clinical applicability of different types of real-time RT-PCR tests in a given group size remains inconclusive due to inconsistent regional disease prevalence and test demands.
Methods: In this study, the performance of one dual-target conventional and two point-of-care real-time RT-PCR tests in a 5-specimen pooled testing strategy for the detection of SARS-COV-2 was evaluated.
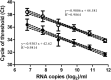
Results: We demonstrated the proof of concept that all of these real-time RT-PCR tests could feasibly detect SARS-CoV-2 from nasopharyngeal and oropharyngeal specimens that contain viral RNA loads in the range of 3.48 × 105 to 3.42 × 102 copies/ml through pooled testing in a group size of 5 with overall positive percent agreement (pooling vs. individual testing) ranging from 100% to 93.75%. Furthermore, the two POC real-time RT-PCR tests exhibited comparable sensitivity to that of the dual-target conventional one when clinical specimens were tested individually.
Conclusion: Our findings support the feasibility of using real-time RT-PCR tests developed as a variety of platforms in routine laboratory detection of suspected COVID-19 cases through a pooled testing strategy that is beneficial to increasing the daily diagnostic capacity.
Keywords: SARS-CoV-2; point-of-care; pooled testing; real-time RT-PCR.
© 2022 The Authors. Journal of Clinical Laboratory Analysis published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Pooled-Testing for SARS-CoV-2 Reverse Transcription Polymerase Chain Reaction (RT-PCR) in asymptomatic healthcare workers in EL-Raghy isolation COVID-19 hospital, Assiut University.Egypt J Immunol. 2022 Apr;29(2):68-75. Egypt J Immunol. 2022. PMID: 35436056
-
Clinical evaluation of a multiplex real-time RT-PCR assay for detection of SARS-CoV-2 in individual and pooled upper respiratory tract samples.Arch Virol. 2021 Sep;166(9):2551-2561. doi: 10.1007/s00705-021-05148-1. Epub 2021 Jul 14. Arch Virol. 2021. PMID: 34259914 Free PMC article.
-
Evaluation of performance of two SARS-CoV-2 Rapid IgM-IgG combined antibody tests on capillary whole blood samples from the fingertip.PLoS One. 2020 Sep 17;15(9):e0237694. doi: 10.1371/journal.pone.0237694. eCollection 2020. PLoS One. 2020. PMID: 32941461 Free PMC article.
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2020 Sep 30;9:CD013639. doi: 10.1002/14651858.CD013639.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Nov 26;11:CD013639. doi: 10.1002/14651858.CD013639.pub3. PMID: 32997361 Updated.
-
Miniaturized Real-Time PCR systems for SARS-CoV-2 detection at the Point-of-Care.Clin Chim Acta. 2022 Nov 1;536:104-111. doi: 10.1016/j.cca.2022.09.014. Epub 2022 Sep 17. Clin Chim Acta. 2022. PMID: 36126763 Free PMC article. Review.
Cited by
-
Effect of swab pooling on the Accula point-of-care RT-PCR for SARS-CoV-2 detection.Front Microbiol. 2023 Aug 7;14:1219214. doi: 10.3389/fmicb.2023.1219214. eCollection 2023. Front Microbiol. 2023. PMID: 37608952 Free PMC article.
-
Evaluation of Rapid Multiplex Reverse Transcription-Quantitative Polymerase Chain Reaction Assays for SARS-CoV-2 Detection in Individual and Pooled Samples.Life (Basel). 2023 Aug 10;13(8):1717. doi: 10.3390/life13081717. Life (Basel). 2023. PMID: 37629574 Free PMC article.
References
-
- WHO . Recommendations for national SARS‐CoV‐2 testing strategies and diagnostic capacities. 2021. Accessed October 14, 2021.https://apps.who.int/iris/bitstream/handle/10665/342002/WHO‐2019‐nCoV‐la...
-
- Sharfstein JM, Becker SJ, Mello MM. Diagnostic testing for the novel coronavirus. JAMA. 2020;323:1437‐1438. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

