Effects of N-acetylcysteine and metformin treatment on the stereopathological characteristics of uterus and ovary
- PMID: 35535444
- PMCID: PMC9295164
- DOI: 10.4081/ejtm.2022.10409
Effects of N-acetylcysteine and metformin treatment on the stereopathological characteristics of uterus and ovary
Abstract
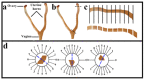
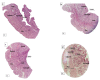
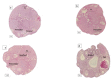
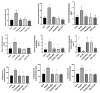
In this study, the stereo-pathological effect of metformin and N-acetyl cysteine is evaluated on the uterus and ovary of polycystic ovary syndrome (PCOS) mice. 96 mature females (8-weekold, weight of 20-30 gr) BALB/c mice were classified into 6 groups including the control group (n= 16), letrozole-induced PCOS group (n=16), PCOS + metformin (n=16), PCOS+NAC (n=16) and a separate control group for NAC (n=16). Another PCOS group was maintained for a month to make sure that features remain till the end of the study. Testosterone level, vaginal cytology and stereological evaluations were assessed. Vaginal cytology in letrozole-receiving mice showed a diestrus phase continuity. Testosterone level, body weight, uterine weight, endometrial volume, myometrial volume, gland volume, stromal volume, epithelial volume, vessel volume, daughter and conglomerate glands, endometrial thickness, and myometrial thickness exhibited an increasing trend in the uterus of PCOS mice. While normal gland and vessel length decreased in the PCOS group. Ovarian volume, corticomedullary volume, primary follicles, secondary follicles, and ovarian cysts were increased in PCOS ovaries. While corpus luteum, primordial, graafian, and atretic follicles showed a decline in the PCOS group. NAC and metformin, however, managed to restore the condition to normal. Given the prevalence of PCOS and its impact on fertility, the use of noninvasive methods is of crucial significance. NAC can control and treat pathological parameters and help as a harmless drug in the treatment of women with PCOS.
Conflict of interest statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Polycystic ovary syndrome (PCOS) is a common hormonal disorder in reproductive age. According to Rotterdam criteria, this syndrome can be diagnosed by two of the three symptoms of clinical/biochemical hyperandrogenism, ovarian dysfunction, and the presence of 12 or more follicles of 2-10 mm in the ovary. Although the number of oocytes retrieved in PCOS is higher than the others, most of them are not mature, declining the pregnancy rate. Oocyte, embryo quality, and pregnancy outcomes are weaker in PCOS patients due to changes in the oocyte and follicular fluid microenvironment. Pathological variations of ovaries and uterus in PCOS are the most important causes of ART failure. Uterine exposure with high levels of free insulin, growth factor-׀, and androgens, increases endometrial proliferative activity, and endometrial thickness. The incidence of micropolip is also associated with endometritis (plasma cells infiltration into endometrial stroma), endometrial stromal edema, and peri-glandular hyperemia. In the ovary, the presence of cystic follicles with a thin layer of granulosa cells and the absence of corpus luteum (indicative of anovulation status) is a common finding. One of the most basic treatment lines of PCOS is metformin. Metformin is a biguanide used to treat hyperglycemia, reduce insulin resistance, and inhibit hepatic gluconeogenesis. It also reduces total and free blood androgen, corrects ovarian dysfunction, and increases the quality of oocyte and embryos by reducing ROS and apoptosis levels. Metformin consumption in PCOS patients can decline premature and pre-antral follicles. Metformin consumption can also enhance follicular development, improve the percentage of corpus luteum, and decrease cystic follicles, hence, facilitating ovulation and fertility rate. Metformin also increments endometrial receptivity markers in PCOS patients. Metformin is, however, associated with gastrointestinal side effects. Another treatment that may help reduce PCOS complications and ultimately increase the chances of fertility is N-Acetyl cysteine (NAC). NAC is a derivative of the amino acid cysteine with antioxidant effects. Its anti-apoptotic properties prevent ovarian ischemia. NAC consumption is associated with a decrease in testosterone. Therefore, NAC can have positive effects on ovulation in PCOS patients. NAC can effectively strengthen pregnancy and ovulation rates. It is safe with a high lethal dose threshold, resulting in no side effects. However, the underlying mechanism of how NAC administration protects the utero-ovarian function (that reverse histopathological characteristics in PCOS) still needs further exploration. In this regard, the present study is aimed to evaluate the stereo pathological effects of metformin and N-acetyl cysteine on uterus and ovary in letrozole-induced PCOS mice to find probable mechanisms.
Figures



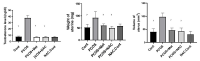
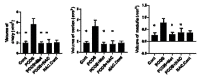
Similar articles
-
The effects of melatonin and metformin on histological characteristics of the ovary and uterus in letrozole-induced polycystic ovarian syndrome mice: A stereological study.Int J Reprod Biomed. 2022 Dec 10;20(11):973-988. doi: 10.18502/ijrm.v20i11.12365. eCollection 2022 Nov. Int J Reprod Biomed. 2022. PMID: 36618831 Free PMC article.
-
The effects of thylakoid-rich spinach extract and aqueous extract of caraway (Carum carvi L.) in letrozole-induced polycystic ovarian syndrome rats.BMC Complement Med Ther. 2020 Aug 12;20(1):249. doi: 10.1186/s12906-020-03044-w. BMC Complement Med Ther. 2020. PMID: 32787839 Free PMC article.
-
Chronic hyperandrogenemia in the presence and absence of a western-style diet impairs ovarian and uterine structure/function in young adult rhesus monkeys.Hum Reprod. 2018 Jan 1;33(1):128-139. doi: 10.1093/humrep/dex338. Hum Reprod. 2018. PMID: 29190387 Free PMC article.
-
AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS, AMERICAN COLLEGE OF ENDOCRINOLOGY, AND ANDROGEN EXCESS AND PCOS SOCIETY DISEASE STATE CLINICAL REVIEW: GUIDE TO THE BEST PRACTICES IN THE EVALUATION AND TREATMENT OF POLYCYSTIC OVARY SYNDROME - PART 2.Endocr Pract. 2015 Dec;21(12):1415-26. doi: 10.4158/EP15748.DSCPT2. Endocr Pract. 2015. PMID: 26642102 Review.
-
Oxidative Stress in Polycystic Ovarian Syndrome and the Effect of Antioxidant N-Acetylcysteine on Ovulation and Pregnancy Rate.Cureus. 2021 Sep 11;13(9):e17887. doi: 10.7759/cureus.17887. eCollection 2021 Sep. Cureus. 2021. PMID: 34660086 Free PMC article. Review.
Cited by
-
Multiple Benefits of Empagliflozin in PCOS: Evidence from a Preclinical Rat Model.Pathophysiology. 2024 Oct 9;31(4):559-582. doi: 10.3390/pathophysiology31040041. Pathophysiology. 2024. PMID: 39449523 Free PMC article.
-
N-acetylcysteine supplementation improves endocrine-metabolism profiles and ovulation induction efficacy in polycystic ovary syndrome.J Ovarian Res. 2024 Oct 16;17(1):205. doi: 10.1186/s13048-024-01528-8. J Ovarian Res. 2024. PMID: 39415242 Free PMC article. Clinical Trial.
-
Phytochemicals-based investigation of Rubia cordifolia pharmacological potential against letrozole-induced polycystic ovarian syndrome in female adult rats: In vitro, in vivo and mechanistic approach.Heliyon. 2024 Jul 9;10(14):e34298. doi: 10.1016/j.heliyon.2024.e34298. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39108850 Free PMC article.
-
Protective effects of vanillic acid on letrozole-induced polycystic ovarian syndrome: A comprehensive study in female wistar rats.Saudi Pharm J. 2024 Feb;32(2):101953. doi: 10.1016/j.jsps.2024.101953. Epub 2024 Jan 9. Saudi Pharm J. 2024. PMID: 38288132 Free PMC article.
-
Effects of N Acetylcysteine on the Expression of Genes Associated with Reproductive Performance in the Goat Uterus during Early Gestation.Animals (Basel). 2022 Sep 15;12(18):2431. doi: 10.3390/ani12182431. Animals (Basel). 2022. PMID: 36139290 Free PMC article.
References
-
- Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nature Reviews Endocrinology. 2018;14(5):270-84. doi: 10.1038/nrendo.2018.24. Epub 2018 Mar 23. - PubMed
-
- Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, et al. . Polycystic ovary syndrome. Nature reviews Disease primers. 2016;2(1):1-18. doi: 10.1038/nrdp.2016.57. - PubMed
-
- Amato G, Conte M, Mazziotti G, Lalli E, Vitolo G, Tucker AT, et al. . Serum and follicular fluid cytokines in polycystic ovary syndrome during stimulated cycles. Obstetrics & Gynecology. 2003;101(6):1177-82. doi: 10.1016/s0029-7844(03)00233-3. - PubMed
-
- Indhavivadhana S, Rattanachaiyanont M, Wongwananuruk T, Techatraisak K, Tanmahasamut P, Dangrat C. Brief communication (Original). Hyperandrogenemia is associated with thin endometrium in reproductiveaged Thai women with polycystic ovary syndrome. Asian Biomedicine. 2013;7(4):545-51.
-
- Kitaya K, Matsubayashi H, Yamaguchi K, Nishiyama R, Takaya Y, Ishikawa T, et al. . Chronic endometritis: potential cause of infertility and obstetric and neonatal complications. American Journal of Reproductive Immunology. 2016;75(1):13-22. doi: 10.1111/aji.12438. Epub 2015 Oct 18. - PubMed
LinkOut - more resources
Full Text Sources

