Efficacy of flukicides on Fasciola gigantica, a food-borne zoonotic helminth affecting livestock in Bangladesh
- PMID: 35535471
- PMCID: PMC11010523
- DOI: 10.1017/S0031182022000580
Efficacy of flukicides on Fasciola gigantica, a food-borne zoonotic helminth affecting livestock in Bangladesh
Abstract
Fasciola gigantica, the causative agent of tropical fasciolosis, is a food-borne zoonotic trematode that affects around 80% livestock of Bangladesh. Triclabendazole (TCBZ), nitroxynil (NTON) and oxyclozanide (OCZN) are frequently used against fascioliasis; however, the current status of potency of these flukicides was unknown. In this study, in vitro efficacy of TCBZ, NTON and OCZN at various concentrations on F. gigantica has been evaluated by relative motility (RM), morphological distortions of apical cone through an inverted microscope, architectural and ultra-structural changes through histopathological and scanning electron microscopy (SEM). It is observed that TCBZ, NTON and OCZN at higher concentrations significantly (P < 0.05) reduced RM of the flukes compared to untreated control. NTON at 150 μg mL−1 was the most potent to reduce the motility within 4 h whereas TCBZ and OCZN were much delayed. Histopathological changes showed swollen, extensive cracking, numerous vacuoles and splitting of the tegument surrounding the spines; spine dislodged from its socket in treated flukes compared to untreated worms. Histopathological changes were more conspicuous at higher doses of TCBZ, NTON and OCZN. SEM has shown the disruption of the apical cone, apart from swelling of the tegument on the ventral surface corrugation and disruption of the ventral apical cone. All these changes indicate that NTON is the most potent in killing flukes in vitro among the tested flukicides and suggest the presence of TCBZ-resistant fluke populations in Bangladesh. It is imperative to explore the in vivo effects of these flukicides and subsequently their molecular mechanisms.
Keywords: Bangladesh; Fasciola gigantica; flukicides; food-borne trematode; in vitro; scanning electron microscopy.
Conflict of interest statement
The authors declare there are no conflicts of interests.
Figures
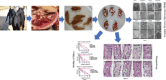
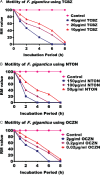



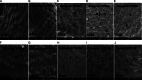
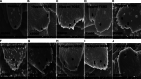
Similar articles
-
Immature Fasciola gigantica: Time-dependent ultrastructural changes following in vivo treatment with triclabendazole.Acta Trop. 2019 Aug;196:15-21. doi: 10.1016/j.actatropica.2019.04.015. Epub 2019 Apr 24. Acta Trop. 2019. PMID: 31028722
-
Fasciola hepatica: a comparative survey of adult fluke resistance to triclabendazole, nitroxynil and closantel on selected upland and lowland sheep farms in Northern Ireland using faecal egg counting, coproantigen ELISA testing and fluke histology.Vet Parasitol. 2015 Jan 15;207(1-2):34-43. doi: 10.1016/j.vetpar.2014.11.016. Epub 2014 Nov 24. Vet Parasitol. 2015. PMID: 25529143
-
Time-dependent tegumental surface changes in juvenile Fasciola gigantica in response to triclabendazole treatment in goat.Acta Trop. 2014 Aug;136:108-17. doi: 10.1016/j.actatropica.2014.04.011. Epub 2014 Apr 15. Acta Trop. 2014. PMID: 24742909
-
Current Threat of Triclabendazole Resistance in Fasciola hepatica.Trends Parasitol. 2016 Jun;32(6):458-469. doi: 10.1016/j.pt.2016.03.002. Epub 2016 Apr 2. Trends Parasitol. 2016. PMID: 27049013 Review.
-
Understanding triclabendazole resistance.Exp Mol Pathol. 2007 Apr;82(2):104-9. doi: 10.1016/j.yexmp.2007.01.009. Epub 2007 Feb 15. Exp Mol Pathol. 2007. PMID: 17398281 Review.
Cited by
-
Foodborne trematodes: old foes, new kids on the block and research perspectives for control and understanding host-parasite interactions.Parasitology. 2022 Sep;149(10):1257-1261. doi: 10.1017/S0031182022000877. Epub 2022 Jun 23. Parasitology. 2022. PMID: 35734871 Free PMC article.
-
An insight into the epidemiology of foodborne zoonotic fascioliasis in small ruminants in northwestern region of Bangladesh.J Parasit Dis. 2024 Jun;48(2):336-346. doi: 10.1007/s12639-024-01672-4. Epub 2024 Apr 29. J Parasit Dis. 2024. PMID: 38840875 Free PMC article.
References
-
- Aghayan S, Gevorgian H, Ebi D, Atoyan HA, Addy F, Mackenstedt U, Romig T and Wassermann M (2019) Fasciola spp. in Armenia: genetic diversity in a global context. Veterinary Parasitology 268, 21–31. - PubMed
-
- Ahasan SA, Valero MA, Chowdhury EH, Islam MT, Islam MR, Mondal MMH, Peixoto RV, Berinde L, Panova M and Mas-Coma S (2016) CIAS detection of Fasciola hepatica/F. gigantica intermediate forms in bovines from Bangladesh. Acta Parasitologica 61, 267–277. - PubMed
-
- Aktaruzzaman M, Mohamed Z, Naim-Ul-Alam M, Siddiqul Islam M and Howlader MR (2015) Evaluation of anthelmintic efficacy of triclabendazole, nitroxynil and albendazole against naturally acquired fascioliasis in cattle of Bangladesh with special reference to its residual effect. International Journal of Pharmacology and Toxicology 3, 1–4.
-
- Amin MR and Samad MA (1988) Clinico-therapeutic studies on bovine fascioliasis. Bangladesh Veterinarian 5, 20–22.
-
- Anderson HR and Fairweather I (1988) Fasciola hepatica: scanning electron microscopic observations of juvenile flukes following treatment in vitro with the deacetylated (amine) metabolite of diamphenethide (DAMD). International Journal for Parasitology 18, 827–837. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

