Clinician perspectives on clinical decision support systems in lung cancer: Implications for shared decision-making
- PMID: 35535474
- PMCID: PMC9327823
- DOI: 10.1111/hex.13457
Clinician perspectives on clinical decision support systems in lung cancer: Implications for shared decision-making
Abstract
Background: Lung cancer treatment decisions are typically made among clinical experts in a multidisciplinary tumour board (MTB) based on clinical data and guidelines. The rise of artificial intelligence and cultural shifts towards patient autonomy are changing the nature of clinical decision-making towards personalized treatments. This can be supported by clinical decision support systems (CDSSs) that generate personalized treatment information as a basis for shared decision-making (SDM). Little is known about lung cancer patients' treatment decisions and the potential for SDM supported by CDSSs. The aim of this study is to understand to what extent SDM is done in current practice and what clinicians need to improve it.
Objective: To explore (1) the extent to which patient preferences are taken into consideration in non-small-cell lung cancer (NSCLC) treatment decisions; (2) clinician perspectives on using CDSSs to support SDM.
Design: Mixed methods study consisting of a retrospective cohort study on patient deviation from MTB advice and reasons for deviation, qualitative interviews with lung cancer specialists and observations of MTB discussions and patient consultations.
Setting and participants: NSCLC patients (N = 257) treated at a single radiotherapy clinic and nine lung cancer specialists from six Dutch clinics.
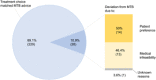
Results: We found a 10.9% (n = 28) deviation rate from MTB advice; 50% (n = 14) were due to patient preference, of which 85.7% (n = 12) chose a less intensive treatment than MTB advice. Current MTB recommendations are based on clinician experience, guidelines and patients' performance status. Most specialists (n = 7) were receptive towards CDSSs but cited barriers, such as lack of trust, lack of validation studies and time. CDSSs were considered valuable during MTB discussions rather than in consultations.
Conclusion: Lung cancer decisions are heavily influenced by clinical guidelines and experience, yet many patients prefer less intensive treatments. CDSSs can support SDM by presenting the harms and benefits of different treatment options rather than giving single treatment advice. External validation of CDSSs should be prioritized.
Patient or public contribution: This study did not involve patients or the public explicitly; however, the study design was informed by prior interviews with volunteers of a cancer patient advocacy group. The study objectives and data collection were supported by Dutch health care insurer CZ for a project titled 'My Best Treatment' that improves patient-centeredness and the lung cancer patient pathway in the Netherlands.
Keywords: clinical decision support systems; lung cancer; multidisciplinary tumour board; patient preferences; patient-centred care; shared decision-making.
© 2022 The Authors. Health Expectations published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures
References
-
- Torre LA, Siegel RL, Jemal A. Lung cancer statistics. In: Ahmad A, Gadgeel S, eds. Lung Cancer and Personalized Medicine. Vol 893. Springer International Publishing; 2016:1‐19. - PubMed
-
- Howlader N, Noone A, Krapcho M. National Cancer Institute. SEER Cancer Statistics Review: 1975‐2011. 2015.
-
- Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389:299‐311. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical