New cytogenetic data on Caryophyllaeus laticeps and Paracaryophyllaeus gotoi, parasites of evolutionary interest
- PMID: 35535487
- PMCID: PMC11010498
- DOI: 10.1017/S0031182022000622
New cytogenetic data on Caryophyllaeus laticeps and Paracaryophyllaeus gotoi, parasites of evolutionary interest
Abstract
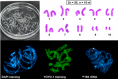
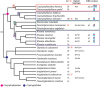
Caryophyllideans are intestinal parasites of freshwater fishes, occupying a basal position among the ‘true’ tapeworms. We performed detailed cytogenetic analyses of the well-known caryophyllidean species Caryophyllaeus laticeps. For comparison, we also examined for the first time the chromosomes of Paracaryophyllaeus gotoi, a specific parasite of loaches in China. Both species showed a diploid chromosome number of 2n = 20, n = 10m. Chromomycin A3 (CMA3)/diamidino-2-phenylindole (DAPI) staining performed for the first time in the class Cestoda revealed CMA3+/DAPI− bands in the pericentromeric regions of the short arms of chromosome pair no. 7 in the karyotype of C. laticeps. Fluorescence in situ hybridization with the 18S rDNA probe confirmed the presence of a single cluster of major rDNA near the centromere on a pair of small chromosomes in both species. These findings support the hypothesis that the ancestral state in the family Caryophyllaeidae is a single interstitial cluster of major rDNA genes and thus one nucleolar organizer region per haploid genome. Our results, which we presented together with literature data plotted on a phylogenetic tree, show stability of caryophyllidean karyotypes at the genus level, but showed differences between genera without a clear phylogenetic signal. The data allowed us to at least formulate a hypothesis about the ancestral haploid chromosome number of n = 10 for the family Caryophyllaeidae and possibly for the sister family Capingentidae. In addition, we compared two populations of C. laticeps from water bodies with different levels of polychlorinated biphenyl contamination, showing a slightly increased incidence of chromosomal abnormalities at the contaminated site.
Keywords: Chromosome aberration; FISH; environmental pollution; karyotype; karyotype evolution; ribosomal DNA.
Conflict of interest statement
The authors declare there are no conflicts of interest.
Figures






References
-
- Bajer A (1959) Change of length and volume of mitotic chromosomes in living cells. Hereditas 45, 579–596.
-
- Barčák D, Oros M, Hanzelová V and Scholz T (2017) A synoptic review of Caryophyllaeus Gmelin, 1790 (Cestoda: Caryophyllidea), parasites of cyprinid fishes. Folia Parasitologica 64, 027. - PubMed
-
- Bazsalovicsová E, Kráľová-Hromadová I, Brabec J, Hanzelová V, Oros M and Scholz T (2014) Conflict between morphology and molecular data: a case of the genus Caryophyllaeus (Cestoda, Caryophyllidea), monozoic tapeworm of cyprinid fishes. Folia Parasitologica 61, 346–352. - PubMed
-
- Bombarová M and Špakulová M (2015) New chromosome characteristics of the monozoic tapeworm Caryophyllaeus laticeps (Cestoda, Caryophyllidea). Helminthologia 52, 336–340.
-
- Brázová T, Miklisová D, Barčák D, Uhrovič D, Šalamún P, Orosová M and Oros M (2021) Hazardous pollutants in the environment: fish host–parasite interactions and bioaccumulation of polychlorinated biphenyls. Environmental Pollution 291, 118175. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

