Early life infection and proinflammatory, atherogenic metabolomic and lipidomic profiles in infancy: a population-based cohort study
- PMID: 35535496
- PMCID: PMC9090335
- DOI: 10.7554/eLife.75170
Early life infection and proinflammatory, atherogenic metabolomic and lipidomic profiles in infancy: a population-based cohort study
Abstract
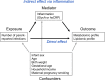
Background: The risk of adult onset cardiovascular and metabolic (cardiometabolic) disease accrues from early life. Infection is ubiquitous in infancy and induces inflammation, a key cardiometabolic risk factor, but the relationship between infection, inflammation, and metabolic profiles in early childhood remains unexplored. We investigated relationships between infection and plasma metabolomic and lipidomic profiles at age 6 and 12 months, and mediation of these associations by inflammation.
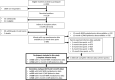
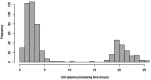
Methods: Matched infection, metabolomics, and lipidomics data were generated from 555 infants in a pre-birth longitudinal cohort. Infection data from birth to 12 months were parent-reported (total infections at age 1, 3, 6, 9, and 12 months), inflammation markers (high-sensitivity C-reactive protein [hsCRP]; glycoprotein acetyls [GlycA]) were quantified at 12 months. Metabolic profiles were 12-month plasma nuclear magnetic resonance metabolomics (228 metabolites) and liquid chromatography/mass spectrometry lipidomics (776 lipids). Associations were evaluated with multivariable linear regression models. In secondary analyses, corresponding inflammation and metabolic data from birth (serum) and 6-month (plasma) time points were used.
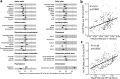
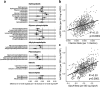
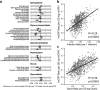
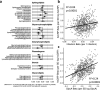
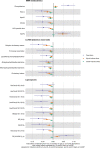
Results: At 12 months, more frequent infant infections were associated with adverse metabolomic (elevated inflammation markers, triglycerides and phenylalanine, and lower high-density lipoprotein [HDL] cholesterol and apolipoprotein A1) and lipidomic profiles (elevated phosphatidylethanolamines and lower trihexosylceramides, dehydrocholesteryl esters, and plasmalogens). Similar, more marked, profiles were observed with higher GlycA, but not hsCRP. GlycA mediated a substantial proportion of the relationship between infection and metabolome/lipidome, with hsCRP generally mediating a lower proportion. Analogous relationships were observed between infection and 6-month inflammation, HDL cholesterol, and apolipoprotein A1.
Conclusions: Infants with a greater infection burden in the first year of life had proinflammatory and proatherogenic plasma metabolomic/lipidomic profiles at 12 months of age that in adults are indicative of heightened risk of cardiovascular disease, obesity, and type 2 diabetes. These findings suggest potentially modifiable pathways linking early life infection and inflammation with subsequent cardiometabolic risk.
Funding: The establishment work and infrastructure for the BIS was provided by the Murdoch Children's Research Institute (MCRI), Deakin University, and Barwon Health. Subsequent funding was secured from National Health and Medical Research Council of Australia (NHMRC), The Shepherd Foundation, The Jack Brockhoff Foundation, the Scobie & Claire McKinnon Trust, the Shane O'Brien Memorial Asthma Foundation, the Our Women's Our Children's Fund Raising Committee Barwon Health, the Rotary Club of Geelong, the Minderoo Foundation, the Ilhan Food Allergy Foundation, GMHBA, Vanguard Investments Australia Ltd, and the Percy Baxter Charitable Trust, Perpetual Trustees. In-kind support was provided by the Cotton On Foundation and CreativeForce. The study sponsors were not involved in the collection, analysis, and interpretation of data; writing of the report; or the decision to submit the report for publication. Research at MCRI is supported by the Victorian Government's Operational Infrastructure Support Program. This work was also supported by NHMRC Senior Research Fellowships to ALP (1008396); DB (1064629); and RS (1045161) , NHMRC Investigator Grants to ALP (1110200) and DB (1175744), NHMRC-A*STAR project grant (1149047). TM is supported by an MCRI ECR Fellowship. SB is supported by the Dutch Research Council (452173113).
Keywords: Barwon Infant Study; epidemiology; global health; human; infection; inflammation; lipidomic; metabolomic; paediatric.
© 2022, Mansell et al.
Conflict of interest statement
TM TM has received a postdoctoral fellowship from MCRI, is supported by NHMRC funding, has received travel support from MCRI and the University of Melbourne, and received a PhD scholarship from the University of Melbourne, RS, SB, AS, RR, PS, FC, PM No competing interests declared, AP ALP is an unpaid scientific advisor for, and has shares in, Dysrupt Labs. ALP has shares in Prevatex Pty Ltd, MT MLKT has received funding paid to Murdoch Childen's Research Institute (MCRI) from NHMRC, Prota Theraputics, Abbott Nutrition, the Allergy and Immunology Foundation of Australasia, and the National Children's Research Centre of Ireland, and has received internal research funding from MCRI. MLKT is inventor of 2 patents owned by MCRI relating to allergy treatment and a method to induce tolerance to an allergen. MLKT is a member of the Advisory Boards for Pfizer (has received personal fee) and Anaphylaxis & Anaphylaxis Australia, and of allergy/anaphylaxis-related Committees for the World Allergy Organisation, the International Union of Immunological Societies, the Asia Pacific Association of Allergy Asthma and Clinical Immunology, the American Academy of Allergy Asthma and Immunology, and the Australasian Society of Clinical Immunology and Allergy. MLKT is employee of, and has share options in, Prota Therapeutics. MLKT is an Associate Editor for the Journal of Allergy and Clinical Immunology: Global, MO MOH has stocks in Prevatex Pty Ltd, SB S Bekkering has received postdoctoral grants from the Dutch Heart Foundation and the Dutch Research Council, and travel support from the European Society for Atherosclerosis, SR SR is Director of the Lung Foundation Australia. SR has stocks/options in Prevatex Pty Ltd, PV PV is an inventor on a patent relating to the relationship between maternal carriage of Prevotella. copri and offspring allergic disease, and has stocks/options in Prevatex Pty Ltd, DB DB has received an Investigator Grant and Project Grant from the Australian National Health and Medical Research Council (NHMRC)
Figures











Similar articles
-
Cord blood lipid correlation network profiles are associated with subsequent attention-deficit/hyperactivity disorder and autism spectrum disorder symptoms at 2 years: a prospective birth cohort study.EBioMedicine. 2024 Feb;100:104949. doi: 10.1016/j.ebiom.2023.104949. Epub 2024 Jan 9. EBioMedicine. 2024. PMID: 38199043 Free PMC article.
-
Ontogeny of circulating lipid metabolism in pregnancy and early childhood - a longitudinal population study.Elife. 2022 Mar 2;11:e72779. doi: 10.7554/eLife.72779. Elife. 2022. PMID: 35234611 Free PMC article.
-
Towards early risk biomarkers: serum metabolic signature in childhood predicts cardio-metabolic risk in adulthood.EBioMedicine. 2021 Oct;72:103611. doi: 10.1016/j.ebiom.2021.103611. Epub 2021 Oct 7. EBioMedicine. 2021. PMID: 34628356 Free PMC article.
-
Effect of biological sex on human circulating lipidome: An overview of the literature.Atherosclerosis. 2023 Nov;384:117274. doi: 10.1016/j.atherosclerosis.2023.117274. Epub 2023 Sep 22. Atherosclerosis. 2023. PMID: 37743161 Review.
-
Long-term cardiometabolic disease risk in women with PCOS: a systematic review and meta-analysis.Hum Reprod Update. 2020 Nov 1;26(6):942-960. doi: 10.1093/humupd/dmaa029. Hum Reprod Update. 2020. PMID: 32995872 Free PMC article.
Cited by
-
Cord blood lipid correlation network profiles are associated with subsequent attention-deficit/hyperactivity disorder and autism spectrum disorder symptoms at 2 years: a prospective birth cohort study.EBioMedicine. 2024 Feb;100:104949. doi: 10.1016/j.ebiom.2023.104949. Epub 2024 Jan 9. EBioMedicine. 2024. PMID: 38199043 Free PMC article.
-
Implementation of Modern Therapeutic Drug Monitoring and Lipidomics Approaches in Clinical Practice: A Case Study with Colistin Treatment.Pharmaceuticals (Basel). 2024 Jun 7;17(6):753. doi: 10.3390/ph17060753. Pharmaceuticals (Basel). 2024. PMID: 38931420 Free PMC article.
-
Inflammation proteomics datasets in the ALSPAC cohort.Wellcome Open Res. 2024 Feb 6;7:277. doi: 10.12688/wellcomeopenres.18482.2. eCollection 2022. Wellcome Open Res. 2024. PMID: 39268475 Free PMC article.
-
Chewing the fat: How lipidomics is changing our understanding of human health and disease in 2022.Anal Sci Adv. 2023 May 10;4(3-4):104-131. doi: 10.1002/ansa.202300009. eCollection 2023 May. Anal Sci Adv. 2023. PMID: 38715925 Free PMC article. Review.
-
Impact of Distinct Antiandrogen Exposures on the Plasma Metabolome in Feminizing Gender-affirming Hormone Therapy.J Clin Endocrinol Metab. 2024 Oct 15;109(11):2857-2871. doi: 10.1210/clinem/dgae226. J Clin Endocrinol Metab. 2024. PMID: 38609170 Free PMC article. Clinical Trial.
References
-
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

