Thermosynechococcus switches the direction of phototaxis by a c-di-GMP-dependent process with high spatial resolution
- PMID: 35535498
- PMCID: PMC9090330
- DOI: 10.7554/eLife.73405
Thermosynechococcus switches the direction of phototaxis by a c-di-GMP-dependent process with high spatial resolution
Abstract
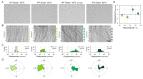
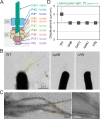
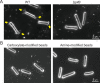
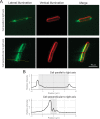
Many cyanobacteria, which use light as an energy source via photosynthesis, show directional movement towards or away from a light source. However, the molecular and cell biological mechanisms for switching the direction of movement remain unclear. Here, we visualized type IV pilus-dependent cell movement in the rod-shaped thermophilic cyanobacterium Thermosynechococcus vulcanus using optical microscopy at physiological temperature and light conditions. Positive and negative phototaxis were controlled on a short time scale of 1 min. The cells smoothly moved over solid surfaces towards green light, but the direction was switched to backward movement when we applied additional blue light illumination. The switching was mediated by three photoreceptors, SesA, SesB, and SesC, which have cyanobacteriochrome photosensory domains and synthesis/degradation activity of the bacterial second messenger cyclic dimeric GMP (c-di-GMP). Our results suggest that the decision-making process for directional switching in phototaxis involves light-dependent changes in the cellular concentration of c-di-GMP. Direct visualization of type IV pilus filaments revealed that rod-shaped cells can move perpendicular to the light vector, indicating that the polarity can be controlled not only by pole-to-pole regulation but also within-a-pole regulation. This study provides insights into previously undescribed rapid bacterial polarity regulation via second messenger signalling with high spatial resolution.
Keywords: infectious disease; microbiology; optical microscopy; photoreceptor; polarity; signal transduction; type IV pili.
Plain language summary
Cyanobacteria, like plants, grow by capturing energy from sunlight. But they have an advantage over their leafy counterparts: they can explore their environment to find the type of light that best suits their needs. These movements rely on hook-like structures, called type IV pili, which allow the cells to pull themselves forward. The pili are usually located at the opposite poles of a rod-shaped cell, allowing the bacteria to move along their longer axis. Yet, the molecular mechanisms that allow cyanobacteria to react to the light are poorly understood. To explore these processes in more detail, Nakane, Enomoto et al. started by shining coloured lights on the rod-shaped cyanobacteria Thermosynechococcus vulcanus. This revealed that the cells moved towards green light but reversed rapidly when blue light was added. The behaviour was disrupted when the genes for three light-sensing proteins were artificially switched off. These molecular players act by changing the levels of cyclic di-GMP, a signalling molecule that may interact with type IV pili. The experiments also showed that T. vulcanus cells were not only moving along their longer axis, but also at a right-angle. This observation contrasts with how other rod-shaped bacteria can explore their environment. A closer look revealed that the cyanobacteria could perform these movements by making asymmetrical adjustment to the way that pili at each pole were working. Further research is now needed to more finely dissect the molecular mechanisms which control this remarkable type of motion.
© 2022, Nakane et al.
Conflict of interest statement
DN, GE, HB, YH, AW, TN No competing interests declared
Figures














References
-
- Auwera GAVd OB. Genomics in the Cloud: Using Docker, GATK, and WDL in Terra / Geraldine A. Sebastopol: Van der Auwera and Brian D. O’Connor. O’Reilly; 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

