Humoral Immunogenicity of the mRNA-1273 Vaccine in the Phase 3 Coronavirus Efficacy (COVE) Trial
- PMID: 35535503
- PMCID: PMC9213865
- DOI: 10.1093/infdis/jiac188
Humoral Immunogenicity of the mRNA-1273 Vaccine in the Phase 3 Coronavirus Efficacy (COVE) Trial
Abstract
Background: Messenger RNA (mRNA)-1273 vaccine demonstrated 93.2% efficacy against coronavirus disease 2019 (COVID-19) in the Coronavirus Efficacy (COVE) trial. The humoral immunogenicity results are now reported.
Methods: Participants received 2 mRNA-1273 (100 µg) or placebo injections, 28 days apart. Immune responses were evaluated in a prespecified, randomly selected per-protocol immunogenicity population (n = 272 placebo; n = 1185 mRNA-1273). Serum binding antibodies (bAbs) and neutralizing antibodies (nAbs) to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-spike protein were assessed at days 1, 29, and 57 by baseline SARS-CoV-2-negative (n = 1197) and SARS-CoV-2-positive (n = 260) status, age, and sex.
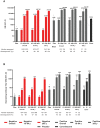
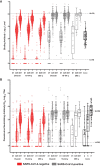
Results: SARS-CoV-2-negative vaccinees had bAb geometric mean AU/mL levels of 35 753 at day 29 that increased to 316 448 at day 57 and nAb inhibitory dilution 50% titers of 55 at day 29 that rose to 1081 at day 57. In SARS-CoV-2-positive vacinees, the first mRNA-1273 injection elicited bAb and nAb levels that were 11-fold (410 049) and 27-fold (1479) higher than in SARS-CoV-2-negative vaccinees, respectively, and were comparable to levels after 2 injections in uninfected participants. Findings were generally consistent by age and sex.
Conclusions: mRNA-1273 elicited robust serologic immune responses across age, sex, and SARS-CoV-2 status, consistent with its high COVID-19 efficacy. Higher immune responses in those previously infected support a booster-type effect. Clinical Trials Registration. NCT04470427.
Keywords: COVE trial; COVID-19; SARS-CoV-2; immunogenicity; mRNA-1273; pseudovirus neutralizing antibody assay; spike-binding antibody assay.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. L. R. B., H. M. E., R. R., and S. S. report grants from NIH and/or NIAID during the conduct of the study. D. M. and A. M. disclose research funding from Moderna. R. P., Y. D. P., B. G., A. A., W. D., H. Z., and B. L. report being employees of Moderna, Inc. and may hold stock/stock options in the company. J. E. T. is a Moderna consultant. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1 AI148575/AI/NIAID NIH HHS/United States
- UM1 AI 68636/NH/NIH HHS/United States
- S10 AI174104/AI/NIAID NIH HHS/United States
- UM1 AI069412/AI/NIAID NIH HHS/United States
- UM1 AI068614/AI/NIAID NIH HHS/United States
- UM1 AI148684/AI/NIAID NIH HHS/United States
- UM1 AI 68635/NH/NIH HHS/United States
- UM1 AI068619/AI/NIAID NIH HHS/United States
- UM1 AI 68614HVTN/NH/NIH HHS/United States
- UM1 AI 68619/NH/NIH HHS/United States
- UM1 AI068618/AI/NIAID NIH HHS/United States
- UM1 AI068635/AI/NIAID NIH HHS/United States
- UL1 TR002384/TR/NCATS NIH HHS/United States
- UM1 AI148684-03/NH/NIH HHS/United States
- UM1 AI 68618/NH/NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI068617/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

