Myeloid Poldip2 Contributes to the Development of Pulmonary Inflammation by Regulating Neutrophil Adhesion in a Murine Model of Acute Respiratory Distress Syndrome
- PMID: 35535614
- PMCID: PMC9238549
- DOI: 10.1161/JAHA.121.025181
Myeloid Poldip2 Contributes to the Development of Pulmonary Inflammation by Regulating Neutrophil Adhesion in a Murine Model of Acute Respiratory Distress Syndrome
Abstract
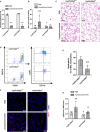
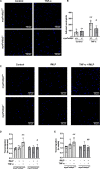
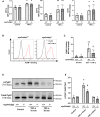
Background Lung injury, a severe adverse outcome of lipopolysaccharide-induced acute respiratory distress syndrome, is attributed to excessive neutrophil recruitment and effector response. Poldip2 (polymerase δ-interacting protein 2) plays a critical role in regulating endothelial permeability and leukocyte recruitment in acute inflammation. Thus, we hypothesized that myeloid Poldip2 is involved in neutrophil recruitment to inflamed lungs. Methods and Results After characterizing myeloid-specific Poldip2 knockout mice, we showed that at 18 hours post-lipopolysaccharide injection, bronchoalveolar lavage from myeloid Poldip2-deficient mice contained fewer inflammatory cells (8 [4-16] versus 29 [12-57]×104/mL in wild-type mice) and a smaller percentage of neutrophils (30% [28%-34%] versus 38% [33%-41%] in wild-type mice), while the main chemoattractants for neutrophils remained unaffected. In vitro, Poldip2-deficient neutrophils responded as well as wild-type neutrophils to inflammatory stimuli with respect to neutrophil extracellular trap formation, reactive oxygen species production, and induction of cytokines. However, neutrophil adherence to a tumor necrosis factor-α stimulated endothelial monolayer was inhibited by Poldip2 depletion (225 [115-272] wild-type [myePoldip2+/+] versus 133 [62-178] myeloid-specific Poldip2 knockout [myePoldip2-/-] neutrophils) as was transmigration (1.7 [1.3-2.1] versus 1.1 [1.0-1.4] relative to baseline transmigration). To determine the underlying mechanism, we examined the surface expression of β2-integrin, its binding to soluble intercellular adhesion molecule 1, and Pyk2 phosphorylation. Surface expression of β2-integrins was not affected by Poldip2 deletion, whereas β2-integrins and Pyk2 were less activated in Poldip2-deficient neutrophils. Conclusions These results suggest that myeloid Poldip2 is involved in β2-integrin activation during the inflammatory response, which in turn mediates neutrophil-to-endothelium adhesion in lipopolysaccharide-induced acute respiratory distress syndrome.
Keywords: ARDS; Poldip2; adhesion; integrin; neutrophil.
Figures


Similar articles
-
Poldip2 deficiency protects against lung edema and vascular inflammation in a model of acute respiratory distress syndrome.Clin Sci (Lond). 2019 Jan 25;133(2):321-334. doi: 10.1042/CS20180944. Print 2019 Jan 31. Clin Sci (Lond). 2019. PMID: 30622219 Free PMC article.
-
Endothelial Poldip2 regulates sepsis-induced lung injury via Rho pathway activation.Cardiovasc Res. 2022 Aug 24;118(11):2506-2518. doi: 10.1093/cvr/cvab295. Cardiovasc Res. 2022. PMID: 34528082 Free PMC article.
-
The proline-rich tyrosine kinase Pyk2 modulates integrin-mediated neutrophil adhesion and reactive oxygen species generation.Biochim Biophys Acta Mol Cell Res. 2020 Oct;1867(10):118799. doi: 10.1016/j.bbamcr.2020.118799. Epub 2020 Jul 18. Biochim Biophys Acta Mol Cell Res. 2020. PMID: 32693110
-
Neutrophils in acute lung injury.Front Biosci (Landmark Ed). 2012 Jun 1;17(6):2278-83. doi: 10.2741/4051. Front Biosci (Landmark Ed). 2012. PMID: 22652778 Review.
-
Neutrophils in the initiation and resolution of acute pulmonary inflammation: understanding biological function and therapeutic potential.J Pathol. 2019 Apr;247(5):672-685. doi: 10.1002/path.5221. Epub 2019 Feb 15. J Pathol. 2019. PMID: 30570146 Free PMC article. Review.
Cited by
-
The polymerase δ-interacting protein family and their emerging roles in diseases.Front Med (Lausanne). 2022 Nov 8;9:1026931. doi: 10.3389/fmed.2022.1026931. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36425112 Free PMC article. Review.
-
Annexin A3 Represses Endothelial Permeability and Inflammation During Sepsis via Actin Cytoskeleton Modulation.Adv Sci (Weinh). 2025 Jun;12(22):e2416904. doi: 10.1002/advs.202416904. Epub 2025 Mar 28. Adv Sci (Weinh). 2025. PMID: 40151887 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

