Myocardial recovery evaluation from ventricular assist device in patients with dilated cardiomyopathy
- PMID: 35535672
- PMCID: PMC9288791
- DOI: 10.1002/ehf2.13951
Myocardial recovery evaluation from ventricular assist device in patients with dilated cardiomyopathy
Abstract
Aims: The removal of left ventricular assist device (LVAD) after myocardial recovery can provide survival benefits with freedom from LVAD-associated complications. However, in the absence of standardization, the weaning evaluation and surgical strategy differ widely among centres. Therefore, we analysed the experiences of LVAD explantation with our protocol in dilated cardiomyopathy (DCM) patients and investigated the validity of our weaning evaluation and surgical strategy from the perspective of optimal long-term survival.
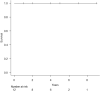
Methods and results: All LVAD explantation patients in our institution between May 2012 and May 2020 were enrolled. All patients were evaluated by our three-phase weaning assessment: (i) clinical stability with improved cardiac function under LVAD support; (ii) haemodynamic stability shown by ramp-loading and saline-loading test; (iii) intraoperative pump-off test. Explant surgery involved removal of the whole system including driveline, pump, sewing ring and outflow-graft, and closure of an apical hole. Intra-operative, peri-operative, and post-operative outcomes, including all-cause mortality and LVAD associated major complications, were retrospectively analysed. A total of 12 DCM patients (DuraHeart, n = 2; EVAHEART, n = 2; HeartMate II, n = 6; HeartMate 3, n = 2) had myocardial recovery after a median 10 months [interquartile range (IQR); 6.3-15 months] support and qualified for our LVAD explantation study protocol [median age: 37 y, IQR; 34-41 years; 83% men]. The median left ventricular ejection fraction was 20% (IQR; 12-23%) at LVAD-implantation and 54% (IQR: 45-55%) before LVAD explantation (P < 0.001). There were no perioperative complications and median ICU stay was 4 days (IQR; 2-4 days). All patients were discharged after a median of 24 days (IQR: 17-28 days) postoperatively. No patient suffered from any cardiac event (heart failure hospitalization, re-implantation of LVAD, or heart transplantation) at a median of 40 months (IQR: 17-58 months) follow up. All patients are alive with NYHA functional class 1 with preserved left ventricular function.
Conclusions: The evaluation of LVAD explant candidates by our weaning protocol was safe and effective. In the patients completing our protocol successfully, LVAD explantation is feasible and an excellent long-term cardiac event free-survival seems to be achieved.
Keywords: Heart failure; LVAD explantation; Left ventricular assist device; Mechanical circulatory support; Weaning protocol.
© 2022 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
All authors declare no conflict of interest in context with this study.
Figures
References
-
- Wever‐Pinzon O, Drakos SG, McKellar SH, Home BD, Caine WT, Horne BD, Kfoury AG, Li DY, Fang JC, Stehlik J, Selzman CH. Cardiac recovery during long‐term left ventricular assist device support. J Am Coll Cardiol. 2016; 68: 1540–1553. - PubMed
-
- Pan S, Aksut B, Wever‐Pinzon OE, Rao SD, Levin AP, Garan AR, Fried JA, Takeda K, Hiroo T, Yuzefpolskaya M, Uriel N, Jorde UP, Mancini DM, Naka Y, Colombo PC, Topkara VK. Incidence and predictors of myocardial recovery on long‐term left ventricular assist device support: Results from the united network for organ sharing database. J Heart Lung Transplant. 2015; 34: 1624–1629. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
