Rescue of behavioral and electrophysiological phenotypes in a Pitt-Hopkins syndrome mouse model by genetic restoration of Tcf4 expression
- PMID: 35535852
- PMCID: PMC9090324
- DOI: 10.7554/eLife.72290
Rescue of behavioral and electrophysiological phenotypes in a Pitt-Hopkins syndrome mouse model by genetic restoration of Tcf4 expression
Abstract
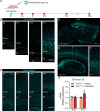
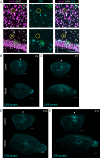
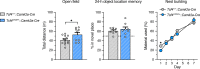
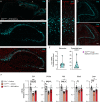
Pitt-Hopkins syndrome (PTHS) is a neurodevelopmental disorder caused by monoallelic mutation or deletion in the transcription factor 4 (TCF4) gene. Individuals with PTHS typically present in the first year of life with developmental delay and exhibit intellectual disability, lack of speech, and motor incoordination. There are no effective treatments available for PTHS, but the root cause of the disorder, TCF4 haploinsufficiency, suggests that it could be treated by normalizing TCF4 gene expression. Here, we performed proof-of-concept viral gene therapy experiments using a conditional Tcf4 mouse model of PTHS and found that postnatally reinstating Tcf4 expression in neurons improved anxiety-like behavior, activity levels, innate behaviors, and memory. Postnatal reinstatement also partially corrected EEG abnormalities, which we characterized here for the first time, and the expression of key TCF4-regulated genes. Our results support a genetic normalization approach as a treatment strategy for PTHS, and possibly other TCF4-linked disorders.
Keywords: Pitt-Hopkins syndrome; gene therapy; genetics; genomics; mouse; neurodevelopmental disorder; neuroscience.
© 2022, Kim et al.
Conflict of interest statement
HK, EG, AD, NB, HV, XZ, AH, KR, JS, AK, BP No competing interests declared
Figures







References
-
- Amiel J, Rio M, de Pontual L, Redon R, Malan V, Boddaert N, Plouin P, Carter NP, Lyonnet S, Munnich A, Colleaux L. Mutations in TCF4, encoding a class I basic helix-loop-helix transcription factor, are responsible for Pitt-Hopkins syndrome, a severe epileptic encephalopathy associated with autonomic dysfunction. American Journal of Human Genetics. 2007;80:988–993. doi: 10.1086/515582. - DOI - PMC - PubMed
-
- Bedeschi MF, Marangi G, Calvello MR, Ricciardi S, Leone FPC, Baccarin M, Guerneri S, Orteschi D, Murdolo M, Lattante S, Frangella S, Keena B, Harr MH, Zackai E, Zollino M. Impairment of different protein domains causes variable clinical presentation within Pitt-Hopkins syndrome and suggests intragenic molecular syndromology of TCF4. European Journal of Medical Genetics. 2017;60:565–571. doi: 10.1016/j.ejmg.2017.08.004. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

