The relationship between amyloid-beta and brain capillary endothelial cells in Alzheimer's disease
- PMID: 35535871
- PMCID: PMC9120708
- DOI: 10.4103/1673-5374.335829
The relationship between amyloid-beta and brain capillary endothelial cells in Alzheimer's disease
Abstract
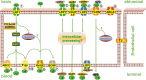
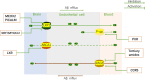
Neurovascular dysfunction, as an integral part of Alzheimer's disease, may have an important influence on the onset and progression of chronic neurodegenerative processes. The blood-brain barrier (BBB) pathway is one of the main pathways that mediates the clearance of amyloid-beta (Aβ) in the brain parenchyma. A large number of studies have shown that receptors and ATP-binding cassette transporters expressed on endothelial cells play an important role in Aβ transport across the BBB, but the specific mechanism is not clear. In this review, we summarize the possible mechanisms of Aβ production and clearance, and in particular the relationship between Aβ and brain capillary endothelial cells. Aβ is produced by abnormal cleavage of the amyloid precursor protein via amyloidogenic processing under pathological conditions. Dysregulation of Aβ clearance is considered to be the main reason for the massive accumulation of Aβ in the brain parenchyma. Several pathways mediating Aβ clearance from the brain into the periphery have been identified, including the BBB pathway, the blood-cerebrospinal fluid barrier and arachnoid granule pathway, and the lymphoid-related pathway. Brain capillary endothelial cells are the key components of Aβ clearance mediated by BBB. Receptors (such as LRP1, RAGE, and FcRn) and ATP-binding cassette transporters (such as P-gp, ABCA1, and ABCC1) expressed on endothelial cells play a critical role in Aβ transcytosis across the BBB. The toxic effects of Aβ can induce dysregulation of receptor and transporter expression on endothelial cells. Excessive Aβ exerts potent detrimental cerebrovascular effects by promoting oxidative stress, inducing chronic inflammation, and impairing endothelial structure and functions. All of these are main causes for the reduction in Aβ clearance across the BBB and the accumulation of Aβ in the brain parenchyma. Therefore, studies on the interactions between Aβ and brain capillary endothelial cells, including their receptors and transporters, studies on inhibition of the toxic effects of Aβ on endothelial cells, and studies on promoting the ability of endothelial cells to mediate Aβ clearance may provide new therapeutic strategies for Aβ clearance in Alzheimer's disease.
Keywords: Alzheimer’s disease; Aβclearance; amyloid beta; blood-brain barrier; cerebral amyloid angiopathy; dementia; endothelial cells; oxidative stress; review; therapeutics; transcytosis.
Conflict of interest statement
None
Figures

References
-
- Akanuma S, Ohtsuki S, Doi Y, Tachikawa M, Ito S, Hori S, Asashima T, Hashimoto T, Yamada K, Ueda K, Iwatsubo T, Terasaki T. ATP-binding cassette transporter A1 (ABCA1) deficiency does not attenuate the brain-to-blood efflux transport of human amyloid-beta peptide (1-40) at the blood-brain barrier. Neurochem Int. 2008;52:956–961. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

