Purinergic signaling systems across comparative models of spinal cord injury
- PMID: 35535876
- PMCID: PMC9120689
- DOI: 10.4103/1673-5374.338993
Purinergic signaling systems across comparative models of spinal cord injury
Erratum in
-
Corrigendum: Purinergic signaling systems across comparative models of spinal cord injury.Neural Regen Res. 2023 Mar;18(3):689-696. doi: 10.4103/1673-5374.350234. Neural Regen Res. 2023. PMID: 36018196 Free PMC article.
Abstract
Within the last several decades, the scientific community has made substantial progress in elucidating the complex pathophysiology underlying spinal cord injury. However, despite the many advances using conventional mammalian models, both cellular and axonal regeneration following spinal cord injury have remained out of reach. In this sense, turning to non-mammalian, regenerative species presents a unique opportunity to identify pro-regenerative cues and characterize a spinal cord microenvironment permissive to re-growth. Among the signaling pathways hypothesized to be dysregulated during spinal cord injury is the purinergic signaling system. In addition to its well-known role as energy currency in cells, ATP and its metabolites are small molecule neurotransmitters that mediate many diverse cellular processes within the central nervous system. While our understanding of the roles of the purinergic system following spinal cord injury is limited, this signaling pathway has been implicated in all injury-induced secondary processes, including cellular death, inflammation, reactive gliosis, and neural regeneration. Given that the purinergic system is also evolutionarily conserved between mammalian and non-mammalian species, comparisons of these roles may provide important insights into conditions responsible for recovery success. Here, we compare the secondary processes between key model species and the influence of purinergic signaling in each context. As our understanding of this signaling system and pro-regenerative conditions continues to evolve, so does the potential for the development of novel therapeutic interventions for spinal cord injury.
Keywords: cell death; differenriation; glia; inflammation; neurogenesis; proliferation; purinergic signaling; reactive gliosis; regeneration; spinal cord injury; teleost.
Conflict of interest statement
Figures


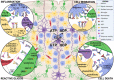

References
-
- Ali AAH, Abdel-Hafiz L, Tundo-Lavalle F, Hassan SA, von Gall C. P2Y2 deficiency impacts adult neurogenesis and related forebrain functions. FASEB J. 2021;35:e21546. - PubMed
-
- Barry D, McDermott K. Differentiation of radial glia from radial precursor cells and transformation into astrocytes in the developing rat spinal cord. Glia. 2005;50:187–197. - PubMed
-
- Becker CG, Becker T, Hugnot JP. The spinal ependymal zone as a source of endogenous repair cells across vertebrates. Prog Neurobiol. 2018;170:67–80. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

