The uPA/uPAR system in astrocytic wound healing
- PMID: 35535878
- PMCID: PMC9120704
- DOI: 10.4103/1673-5374.338991
The uPA/uPAR system in astrocytic wound healing
Abstract
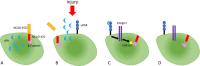
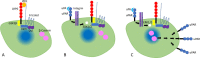
The repair of injured tissue is a highly complex process that involves cell proliferation, differentiation, and migration. Cell migration requires the dismantling of intercellular contacts in the injured zone and their subsequent reconstitution in the wounded area. Urokinase-type plasminogen activator (uPA) is a serine proteinase found in multiple cell types including endothelial cells, smooth muscle cells, monocytes, and macrophages. A substantial body of experimental evidence with different cell types outside the central nervous system indicates that the binding of uPA to its receptor (uPAR) on the cell surface prompts cell migration by inducing plasmin-mediated degradation of the extracellular matrix. In contrast, although uPA and uPAR are abundantly found in astrocytes and uPA binding to uPAR triggers astrocytic activation, it is unknown if uPA also plays a role in astrocytic migration. Neuronal cadherin is a member of cell adhesion proteins pivotal for the formation of cell-cell contacts between astrocytes. More specifically, while the extracellular domain of neuronal cadherin interacts with the extracellular domain of neuronal cadherin in neighboring cells, its intracellular domain binds to β-catenin, which in turn links the complex to the actin cytoskeleton. Glycogen synthase kinase 3β is a serine-threonine kinase that prevents the cytoplasmic accumulation of β-catenin by inducing its phosphorylation at Ser33, Ser37, and Ser41, thus activating a sequence of events that lead to its proteasomal degradation. The data discussed in this perspective indicate that astrocytes release uPA following a mechanical injury, and that binding of this uPA to uPAR on the cell membrane induces the detachment of β-catenin from the intracellular domain of neuronal cadherin by triggering its extracellular signal-regulated kinase 1/2-mediated phosphorylation at Tyr650. Remarkably, this is followed by the cytoplasmic accumulation of β-catenin because uPA-induced extracellular signal-regulated kinase 1/2 activation also phosphorylates lipoprotein receptor-related protein 6 at Ser1490, which in turn, by recruiting glycogen synthase kinase 3β to its intracellular domain abrogates its effect on β-catenin. The cytoplasmic accumulation of β-catenin is followed by its nuclear translocation, where it induces the expression of uPAR, which is required for the migration of astrocytes from the injured edge into the wounded area.
Keywords: Wnt-β-catenin pathway; astrocytes; lipoprotein receptor-related protein 6; plasmin; urokinase receptor; urokinase-type plasminogen activator; wound healing; β-catenin.
Conflict of interest statement
Figures
Similar articles
-
Urokinase-type plasminogen activator-mediated crosstalk between N-cadherin and β-catenin promotes wound healing.J Cell Sci. 2021 Jun 1;134(11):jcs255919. doi: 10.1242/jcs.255919. Epub 2021 Jun 4. J Cell Sci. 2021. PMID: 34085693 Free PMC article.
-
A Cross Talk between Neuronal Urokinase-type Plasminogen Activator (uPA) and Astrocytic uPA Receptor (uPAR) Promotes Astrocytic Activation and Synaptic Recovery in the Ischemic Brain.J Neurosci. 2017 Oct 25;37(43):10310-10322. doi: 10.1523/JNEUROSCI.1630-17.2017. Epub 2017 Sep 20. J Neurosci. 2017. PMID: 28931568 Free PMC article.
-
Urokinase-Type Plasminogen Activator Triggers Wingless/Int1-Independent Phosphorylation of the Low-Density Lipoprotein Receptor-Related Protein-6 in Cerebral Cortical Neurons.J Alzheimers Dis. 2022;89(3):877-891. doi: 10.3233/JAD-220320. J Alzheimers Dis. 2022. PMID: 35964187
-
Structure, function and expression on blood and bone marrow cells of the urokinase-type plasminogen activator receptor, uPAR.Stem Cells. 1997;15(6):398-408. doi: 10.1002/stem.150398. Stem Cells. 1997. PMID: 9402652 Review.
-
[Mechanism of tumor cell-induced extracellular matrix degradation--inhibition of cell-surface proteolytic activity might have a therapeutic effect on tumor cell invasion and metastasis].Nihon Sanka Fujinka Gakkai Zasshi. 1996 Aug;48(8):623-32. Nihon Sanka Fujinka Gakkai Zasshi. 1996. PMID: 8808830 Review. Japanese.
Cited by
-
Diurnal Oscillations of Fibrinolytic Parameters in Patients with Acute Myocardial Infarction and Their Relation to Platelet Reactivity: Preliminary Insights.J Clin Med. 2022 Nov 30;11(23):7105. doi: 10.3390/jcm11237105. J Clin Med. 2022. PMID: 36498682 Free PMC article.
-
Association Between Circulating Inflammatory Cytokines and Dentofacial Anomalies.Int Dent J. 2025 Apr;75(2):885-897. doi: 10.1016/j.identj.2024.09.005. Epub 2024 Oct 5. Int Dent J. 2025. PMID: 39368924 Free PMC article.
References
-
- Andreasen PA, Kjøller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer. 1997;72:1–22. - PubMed
-
- Beschorner R, Schluesener HJ, Nguyen TD, Magdolen V, Luther T, Pedal I, Mattern R, Meyermann R, Schwab JM. Lesion-associated accumulation of uPAR/CD87- expressing infiltrating granulocytes, activated microglial cells/macrophages and upregulation by endothelial cells following TBI and FCI in humans. Neuropathol Appl Neurobiol. 2000;26:522–527. - PubMed
-
- Buck RC. Cell migration in repair of mouse corneal epithelium. Invest Ophthalmol Vis Sci. 1979;18:767–784. - PubMed
-
- Carmeliet P, Moons L, Herbert JM, Crawley J, Lupu F, Lijnen R, Collen D. Urokinase but not tissue plasminogen activator mediates arterial neointima formation in mice. Circ Res. 1997;81:829–839. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

