Human Metapneumovirus Phosphoprotein Independently Drives Phase Separation and Recruits Nucleoprotein to Liquid-Like Bodies
- PMID: 35536005
- PMCID: PMC9239117
- DOI: 10.1128/mbio.01099-22
Human Metapneumovirus Phosphoprotein Independently Drives Phase Separation and Recruits Nucleoprotein to Liquid-Like Bodies
Abstract
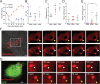
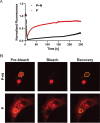
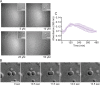
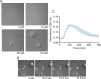
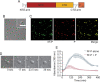
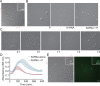
Human metapneumovirus (HMPV) inclusion bodies (IBs) are dynamic structures required for efficient viral replication and transcription. The minimum components needed to form IB-like structures in cells are the nucleoprotein (N) and the tetrameric phosphoprotein (P). HMPV P binds to the following two versions of the N protein in infected cells: N-terminal P residues interact with monomeric N (N0) to maintain a pool of protein to encapsidate new RNA and C-terminal P residues interact with oligomeric, RNA-bound N (N-RNA). Recent work on other negative-strand viruses has suggested that IBs are, at least in part, liquid-like phase-separated membraneless organelles. Here, HMPV IBs in infected or transfected cells were shown to possess liquid organelle properties, such as fusion and fission. Recombinant versions of HMPV N and P proteins were purified to analyze the interactions required to drive phase separation in vitro. Purified HMPV P was shown to form liquid droplets in isolation. This observation is distinct from other viral systems that also form IBs. Partial removal of nucleic acid from purified P altered phase-separation dynamics, suggesting that nucleic acid interactions play a role in IB formation. HMPV P also recruits monomeric N (N0-P) and N-RNA to droplets in vitro. These findings suggest that HMPV P may also act as a scaffold protein to mediate multivalent interactions with monomeric and oligomeric N, as well as RNA, to promote phase separation of IBs. Together, these findings highlight an additional layer of regulation in HMPV replication by the viral P and N proteins. IMPORTANCE Human metapneumovirus (HMPV) is a leading cause of respiratory disease among children, immunocompromised individuals, and the elderly. Currently, no vaccines or antivirals are available for the treatment of HMPV infections. Cytoplasmic inclusion bodies (IBs), where HMPV replication and transcription occur, represent a promising target for the development of novel antivirals. The HMPV nucleoprotein (N) and phosphoprotein (P) are the minimal components needed for IB formation in eukaryotic cells. However, interactions that regulate the formation of these dynamic structures are poorly understood. Here, we showed that HMPV IBs possess the properties of liquid organelles and that purified HMPV P phase separates independently in vitro. Our work suggests that HMPV P phase-separation dynamics are altered by nucleic acid. We provide strong evidence that, unlike results reported from other viral systems, HMPV P alone can serve as a scaffold for multivalent interactions with monomeric (N0) and oligomeric (N-RNA) HMPV N for IB formation.
Keywords: HMPV; inclusion bodies; phase separation; pneumovirus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Specific Residues in the C-Terminal Domain of the Human Metapneumovirus Phosphoprotein Are Indispensable for Formation of Viral Replication Centers and Regulation of the Function of the Viral Polymerase Complex.J Virol. 2023 May 31;97(5):e0003023. doi: 10.1128/jvi.00030-23. Epub 2023 Apr 24. J Virol. 2023. PMID: 37092993 Free PMC article.
-
Minimal Elements Required for the Formation of Respiratory Syncytial Virus Cytoplasmic Inclusion Bodies In Vivo and In Vitro.mBio. 2020 Sep 22;11(5):e01202-20. doi: 10.1128/mBio.01202-20. mBio. 2020. PMID: 32963000 Free PMC article.
-
Human Metapneumovirus Induces Formation of Inclusion Bodies for Efficient Genome Replication and Transcription.J Virol. 2017 Nov 30;91(24):e01282-17. doi: 10.1128/JVI.01282-17. Print 2017 Dec 15. J Virol. 2017. PMID: 28978704 Free PMC article.
-
Interactions between the Nucleoprotein and the Phosphoprotein of Pneumoviruses: Structural Insight for Rational Design of Antivirals.Viruses. 2021 Dec 6;13(12):2449. doi: 10.3390/v13122449. Viruses. 2021. PMID: 34960719 Free PMC article. Review.
-
New Perspectives on the Biogenesis of Viral Inclusion Bodies in Negative-Sense RNA Virus Infections.Cells. 2021 Jun 10;10(6):1460. doi: 10.3390/cells10061460. Cells. 2021. PMID: 34200781 Free PMC article. Review.
Cited by
-
Assembly of the Tripartite and RNA Condensates of the Respiratory Syncytial Virus Factory Proteins In Vitro: Role of the Transcription Antiterminator M2-1.Viruses. 2023 Jun 6;15(6):1329. doi: 10.3390/v15061329. Viruses. 2023. PMID: 37376628 Free PMC article.
-
Nipah Virus Impairs Autocrine IFN Signaling by Sequestering STAT1 and STAT2 into Inclusion Bodies.Viruses. 2023 Feb 17;15(2):554. doi: 10.3390/v15020554. Viruses. 2023. PMID: 36851768 Free PMC article.
-
Liaisons dangereuses: Intrinsic Disorder in Cellular Proteins Recruited to Viral Infection-Related Biocondensates.Int J Mol Sci. 2023 Jan 21;24(3):2151. doi: 10.3390/ijms24032151. Int J Mol Sci. 2023. PMID: 36768473 Free PMC article.
-
Spatial and functional arrangement of Ebola virus polymerase inside phase-separated viral factories.Nat Commun. 2023 Jul 13;14(1):4159. doi: 10.1038/s41467-023-39821-7. Nat Commun. 2023. PMID: 37443171 Free PMC article.
-
Properties of rabies virus phosphoprotein and nucleoprotein biocondensates formed in vitro and in cellulo.PLoS Pathog. 2022 Dec 8;18(12):e1011022. doi: 10.1371/journal.ppat.1011022. eCollection 2022 Dec. PLoS Pathog. 2022. PMID: 36480574 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

