Intracellular mono-ADP-ribosyltransferases at the host-virus interphase
- PMID: 35536484
- PMCID: PMC9087173
- DOI: 10.1007/s00018-022-04290-6
Intracellular mono-ADP-ribosyltransferases at the host-virus interphase
Abstract
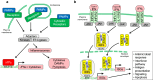
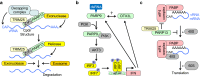
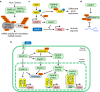
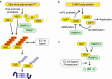
The innate immune system, the primary defense mechanism of higher organisms against pathogens including viruses, senses pathogen-associated molecular patterns (PAMPs). In response to PAMPs, interferons (IFNs) are produced, allowing the host to react swiftly to viral infection. In turn the expression of IFN-stimulated genes (ISGs) is induced. Their products disseminate the antiviral response. Among the ISGs conserved in many species are those encoding mono-ADP-ribosyltransferases (mono-ARTs). This prompts the question whether, and if so how, mono-ADP-ribosylation affects viral propagation. Emerging evidence demonstrates that some mono-ADP-ribosyltransferases function as PAMP receptors and modify both host and viral proteins relevant for viral replication. Support for mono-ADP-ribosylation in virus-host interaction stems from the findings that some viruses encode mono-ADP-ribosylhydrolases, which antagonize cellular mono-ARTs. We summarize and discuss the evidence linking mono-ADP-ribosylation and the enzymes relevant to catalyze this reversible modification with the innate immune response as part of the arms race between host and viruses.
Keywords: ADP-ribosylation; Alphavirus; Chikungunya virus; Coronavirus; Hydrolase; Interferon; MARylation; Macrodomain; PARP; Pattern recognition receptors; Signaling.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

