Comparative Effectiveness Associated With Buprenorphine and Naltrexone in Opioid Use Disorder and Cooccurring Polysubstance Use
- PMID: 35536575
- PMCID: PMC9092203
- DOI: 10.1001/jamanetworkopen.2022.11363
Comparative Effectiveness Associated With Buprenorphine and Naltrexone in Opioid Use Disorder and Cooccurring Polysubstance Use
Abstract
Importance: Despite prevalent polysubstance use, treatment patterns and outcomes for individuals with opioid use disorder (OUD) and cooccurring substance use disorders (SUD) are understudied.
Objective: To evaluate the distribution of buprenorphine and naltrexone initiation among individuals with OUD with vs without cooccurring SUD and to assess the comparative effectiveness associated with buprenorphine and naltrexone against drug-related poisonings.
Design, setting, and participants: This observational comparative effectiveness study used insurance claims from 2011 to 2016 from the US IBM MarketScan databases to study initiation of medications for OUD (MOUD) among treatment-seeking individuals aged 12 to 64 years with a primary diagnosis of OUD. Cooccurring SUD was defined as SUD diagnosed concurrent with or in the 6 months prior to OUD treatment initiation. Treatment was codified as psychosocial treatment without MOUD or initiation or buprenorphine or naltrexone (including extended-release or oral). Methadone recipients were excluded from analysis. Data were analyzed from February 3, 2021, through February 26, 2022.
Exposures: MOUD.
Main outcomes and measures: Associations between cooccurring SUD diagnoses with treatment type were assessed with multivariable regression. The association of drug-related poisoning admissions with days covered with buprenorphine or naltrexone prescriptions vs days without prescriptions was assessed among MOUD initiators. Odds ratios from within-person fixed effects models were estimated as a function of MOUD and stratified by cooccurring SUDs.
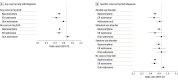
Results: Among 179 280 individuals with OUD (mean [SD] age, 33.2 [11.0] years; 90 196 [50.5%] men), 102 930 (57.4%) received psychosocial treatment without MOUD. Across 47 488 individuals with cooccurring SUDs, 33 449 (70.4%) did not receive MOUD, whereas across 131 792 individuals without cooccurring SUDs, 69 481 (52.7%) did not receive MOUD. Cooccurring SUD was associated with decreased odds of initiating buprenorphine (risk ratio [RR], 0.55 [95% CI, 0.54-0.56]) but increased odds of initiating naltrexone (extended release: RR, 1.12 [95% CI, 1.05-1.20]; oral: RR, 1.95 [95% CI, 1.86-2.03]). Among 12 485 individuals initiating MOUD who experienced at least 1 drug-related poisoning during insurance enrollment, buprenorphine treatment days were associated with decreased poisonings compared with days without MOUD for individuals with cooccurring SUD (odds ratio [OR], 0.56 [95% CI, 0.48-0.65]) and individuals without cooccurring SUD (OR, 0.57 [95% CI, 0.53-0.63]), with comparable associations observed for extended-release naltrexone. No protective association was observed for oral naltrexone.
Conclusions and relevance: These findings suggest that individuals with OUD and polysubstance use were less likely to initiate buprenorphine and naltrexone than individuals without polysubstance use. Among individuals initiating MOUD, polysubstance use was associated with decreased buprenorphine and increased naltrexone initiation, despite buprenorphine's protective associations against drug-related poisoning.
Conflict of interest statement
Figures

Similar articles
-
Pregnancy Rates Among Women Treated with Medication for Opioid Use Disorder.J Gen Intern Med. 2024 Jun;39(8):1342-1348. doi: 10.1007/s11606-024-08689-8. Epub 2024 Feb 29. J Gen Intern Med. 2024. PMID: 38424347 Free PMC article.
-
Association of Opioid Use Disorder Treatment With Alcohol-Related Acute Events.JAMA Netw Open. 2021 Feb 1;4(2):e210061. doi: 10.1001/jamanetworkopen.2021.0061. JAMA Netw Open. 2021. PMID: 33625511 Free PMC article.
-
Association of polysubstance use disorder with treatment quality among Medicaid beneficiaries with opioid use disorder.J Subst Abuse Treat. 2023 Jan;144:108921. doi: 10.1016/j.jsat.2022.108921. Epub 2022 Oct 27. J Subst Abuse Treat. 2023. PMID: 36327615 Free PMC article.
-
Management of opioid withdrawal and initiation of medications for opioid use disorder in the hospital setting.Hosp Pract (1995). 2022 Oct;50(4):251-258. doi: 10.1080/21548331.2022.2102776. Epub 2022 Jul 22. Hosp Pract (1995). 2022. PMID: 35837678 Review.
-
Access to Medications for Opioid Use Disorder and Associated Factors Among Adolescents and Young Adults: A Systematic Review.JAMA Pediatr. 2022 Mar 1;176(3):304-311. doi: 10.1001/jamapediatrics.2021.4606. JAMA Pediatr. 2022. PMID: 34870707 Free PMC article.
Cited by
-
Estimating the impact of stimulant use on initiation of buprenorphine and extended-release naltrexone in two clinical trials and real-world populations.Addict Sci Clin Pract. 2023 Feb 14;18(1):11. doi: 10.1186/s13722-023-00364-3. Addict Sci Clin Pract. 2023. PMID: 36788634 Free PMC article. Clinical Trial.
-
Postmortem toxicology findings from the Camden Opioid Research Initiative.PLoS One. 2023 Nov 1;18(11):e0292674. doi: 10.1371/journal.pone.0292674. eCollection 2023. PLoS One. 2023. PMID: 37910493 Free PMC article.
-
Buprenorphine and postpartum contraception utilization among people with opioid use disorder: a multi-state analysis.Addict Sci Clin Pract. 2025 Jan 6;20(1):1. doi: 10.1186/s13722-024-00530-1. Addict Sci Clin Pract. 2025. PMID: 39762993 Free PMC article.
-
Substance-induced mental disorders and discontinuation of medication for opioid use disorder.Drug Alcohol Depend. 2025 Jul 1;272:112685. doi: 10.1016/j.drugalcdep.2025.112685. Epub 2025 Apr 22. Drug Alcohol Depend. 2025. PMID: 40319789
-
Evolution of the substance use landscape: Implications for contingency management.J Appl Behav Anal. 2025 Jan;58(1):36-55. doi: 10.1002/jaba.2911. Epub 2024 Aug 28. J Appl Behav Anal. 2025. PMID: 39193870 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

