Caffeine intake exerts dual genome-wide effects on hippocampal metabolism and learning-dependent transcription
- PMID: 35536645
- PMCID: PMC9197525
- DOI: 10.1172/JCI149371
Caffeine intake exerts dual genome-wide effects on hippocampal metabolism and learning-dependent transcription
Abstract
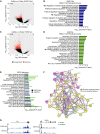
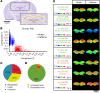
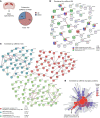
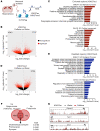
Caffeine is the most widely consumed psychoactive substance in the world. Strikingly, the molecular pathways engaged by its regular consumption remain unclear. We herein addressed the mechanisms associated with habitual (chronic) caffeine consumption in the mouse hippocampus using untargeted orthogonal omics techniques. Our results revealed that chronic caffeine exerts concerted pleiotropic effects in the hippocampus at the epigenomic, proteomic, and metabolomic levels. Caffeine lowered metabolism-related processes (e.g., at the level of metabolomics and gene expression) in bulk tissue, while it induced neuron-specific epigenetic changes at synaptic transmission/plasticity-related genes and increased experience-driven transcriptional activity. Altogether, these findings suggest that regular caffeine intake improves the signal-to-noise ratio during information encoding, in part through fine-tuning of metabolic genes, while boosting the salience of information processing during learning in neuronal circuits.
Keywords: Epigenetics; Memory; Neuroscience; Pharmacology.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

