Tissue-localized immune responses in people with cystic fibrosis and respiratory nontuberculous mycobacteria infection
- PMID: 35536650
- PMCID: PMC9309058
- DOI: 10.1172/jci.insight.157865
Tissue-localized immune responses in people with cystic fibrosis and respiratory nontuberculous mycobacteria infection
Abstract
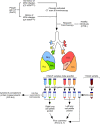
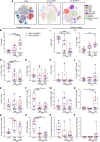
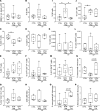
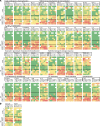
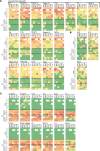
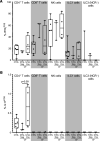
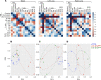
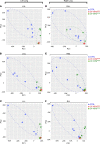
Nontuberculous mycobacteria (NTM) are an increasingly common cause of respiratory infection in people with cystic fibrosis (PwCF). Relative to those with no history of NTM infection (CF-NTMNEG), PwCF and a history of NTM infection (CF-NTMPOS) are more likely to develop severe lung disease and experience complications over the course of treatment. In other mycobacterial infections (e.g., tuberculosis), an overexuberant immune response causes pathology and compromises organ function; however, since the immune profiles of CF-NTMPOS and CF-NTMNEG airways are largely unexplored, it is unknown which, if any, immune responses distinguish these cohorts or concentrate in damaged tissues. Here, we evaluated lung lobe-specific immune profiles of 3 cohorts (CF-NTMPOS, CF-NTMNEG, and non-CF adults) and found that CF-NTMPOS airways are distinguished by a hyperinflammatory cytokine profile. Importantly, the CF-NTMPOS airway immune profile was dominated by B cells, classical macrophages, and the cytokines that support their accumulation. These and other immunological differences between cohorts, including the near absence of NK cells and complement pathway members, were enriched in the most damaged lung lobes. The implications of these findings for our understanding of lung disease in PwCF are discussed, as are how they may inform the development of host-directed therapies to improve NTM disease treatment.
Keywords: Bacterial infections; Cytokines; Infectious disease; Pulmonology; Tuberculosis.
Figures

Similar articles
-
Deficient Complement Opsonization Impairs Mycobacterium avium Killing by Neutrophils in Cystic Fibrosis.Microbiol Spectr. 2023 Feb 14;11(1):e0327922. doi: 10.1128/spectrum.03279-22. Epub 2023 Jan 18. Microbiol Spectr. 2023. PMID: 36651756 Free PMC article.
-
Specificity of Immunoglobulin Response to Nontuberculous Mycobacteria Infection in People with Cystic Fibrosis.Microbiol Spectr. 2022 Aug 31;10(4):e0187422. doi: 10.1128/spectrum.01874-22. Epub 2022 Jul 6. Microbiol Spectr. 2022. PMID: 35863022 Free PMC article.
-
Sputum Metabolites Associated with Nontuberculous Mycobacterial Infection in Cystic Fibrosis.mSphere. 2022 Jun 29;7(3):e0010422. doi: 10.1128/msphere.00104-22. Epub 2022 Apr 28. mSphere. 2022. PMID: 35477313 Free PMC article.
-
Nontuberculous Mycobacterial Infections in Cystic Fibrosis.Clin Chest Med. 2022 Dec;43(4):697-716. doi: 10.1016/j.ccm.2022.06.010. Clin Chest Med. 2022. PMID: 36344075 Review.
-
Nontuberculous mycobacterial infections in cystic fibrosis.Clin Chest Med. 2015 Mar;36(1):101-15. doi: 10.1016/j.ccm.2014.11.003. Clin Chest Med. 2015. PMID: 25676523 Review.
Cited by
-
Nonantibiotic Management of Nontuberculous Mycobacteria in Non-Cystic Fibrosis Bronchiectasis: Natural or Nonsense?Ann Am Thorac Soc. 2024 Apr;21(4):543-545. doi: 10.1513/AnnalsATS.202402-129ED. Ann Am Thorac Soc. 2024. PMID: 38557420 Free PMC article. No abstract available.
-
The Many Hosts of Mycobacteria 9 (MHM9): A conference report.Tuberculosis (Edinb). 2023 Sep;142:102377. doi: 10.1016/j.tube.2023.102377. Epub 2023 Jul 23. Tuberculosis (Edinb). 2023. PMID: 37531864 Free PMC article.
-
IgG Concentrations Distinguish People with Cystic Fibrosis and Mycobacterium abscessus.Lung. 2024 Dec;202(6):845-847. doi: 10.1007/s00408-024-00749-4. Epub 2024 Sep 28. Lung. 2024. PMID: 39340676 No abstract available.
-
Deficient Complement Opsonization Impairs Mycobacterium avium Killing by Neutrophils in Cystic Fibrosis.Microbiol Spectr. 2023 Feb 14;11(1):e0327922. doi: 10.1128/spectrum.03279-22. Epub 2023 Jan 18. Microbiol Spectr. 2023. PMID: 36651756 Free PMC article.
-
Proteomics profiling of inflammatory responses to elexacaftor/tezacaftor/ivacaftor in cystic fibrosis.Front Immunol. 2025 Jan 28;16:1486784. doi: 10.3389/fimmu.2025.1486784. eCollection 2025. Front Immunol. 2025. PMID: 39935472 Free PMC article.

