Six-Month Outcomes of Reimplantation of a Coin-Sized Tibial Nerve Stimulator for the Treatment of Overactive Bladder Syndrome With Urgency Urinary Incontinence
- PMID: 35536667
- PMCID: PMC9071024
- DOI: 10.1097/SPV.0000000000001105
Six-Month Outcomes of Reimplantation of a Coin-Sized Tibial Nerve Stimulator for the Treatment of Overactive Bladder Syndrome With Urgency Urinary Incontinence
Abstract
Objective: The eCoin (Valencia Technologies Corporation, Valencia, CA) is a battery-powered, nickel-sized and shaped neuromodulation device for the treatment of overactive bladder, and it is implanted in the lower leg in a short office or outpatient procedure under local anesthesia. A follow-on trial was conducted to evaluate the feasibility, safety, and efficacy of eCoin reimplantation.
Methods: This was a prospective, single-arm, open-label study, including 23 participants with refractory urgency urinary incontinence (UUI) who were previously participants in the eCoin clinical feasibility trial. This follow-on study was conducted at 7 sites in the United States and New Zealand. Participants were reimplanted with a new eCoin device and activated after 4 weeks. Bladder diary data and validated quality-of-life instruments, collected at 12 weeks and 24 weeks postactivation, were compared with baseline.
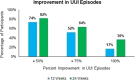
Results: Participants of the study were considered responders if they reported a ≥ 50% reduction from baseline in episodes of UUI on a 3-day voiding diary. At 12 weeks of treatment, 74% (95% confidence interval [CI], 52%-90%) of participants were considered responders. At 24 weeks of treatment, 82% (95% CI, 60%-95%) of participants were considered responders with 36% (95% CI, 20%-57%) of participants achieving complete continence. There were no device-related serious adverse events reported during the study.
Conclusions: The reimplantation of eCoin was both safe and effective in treating UUI associated with overactive bladder syndrome. The demonstrated significant reduction or resolution of symptoms with no serious safety concern suggests that eCoin is a convenient and maintainable therapeutic device.
Trial registration: ClinicalTrials.gov NCT03655054 NCT03029624.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
B.K. declares that he serves as a consultant for Valencia Technologies, was an investigator on this Valencia-sponsored clinical trial, and owns minor equity interest in the company. S.K.S. declares that he has been compensated as a consultant for Valencia Technologies, and owns stock options representing less than 0.01% of the company. The remaining authors have declared they have no conflicts of interest. The authors who own a minor equity interest in the company constitute a very small percentage; all authors constitute less than 0.1% of the company. Furthermore, this potential conflict of interest was addressed according to the recommendations of the local and central IRBs including: (i) Having a statement in the informed consent stating the PI own a minority stake, and disclosing the potential conflict of interest for which the participant has to sign. (ii) The data collected for this study come from a voiding diary and quality of life questionnaires, which are filled out by the participants. The PIs and Clinical Research Coordinators (CRCs) were not allowed to revise or write on these forms. If any formatting edits were made, the participants were asked to change and initial the change themselves. (iii) Clinical Research Coordinators had no conflict of interest and they were the individuals who scanned and sent the raw data.
Figures
References
-
- Choo MS Ku JH Lee JB, et al. . Cross-cultural differences for adapting overactive bladder symptoms: results of an epidemiologic survey in Korea. World J Urol 2007;25:505–511. doi:10.1007/s00345-007-0183-6. - PubMed
-
- Corcos J, Schick E. Prevalence of overactive bladder and incontinence in Canada. Can J Urol 2004;11:2278–2284. - PubMed
-
- Coyne KS Sexton CC Vats V, et al. . National community prevalence of overactive bladder in the United States stratified by sex and age. Urology 2011;77:1081–1087. doi:10.1016/j.urology.2010.08.039. - PubMed
-
- Herschorn S Gajewski J Schulz J, et al. . A population-based study of urinary symptoms and incontinence: the Canadian Urinary Bladder Survey. BJU Int 2008;101:52–58. doi:10.1111/j.1464-410X.2007.07198.x. - PubMed
-
- Irwin DE Milsom I Hunskaar S, et al. . Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 2006;50:1306–1314. doi:10.1016/j.eururo.2006.09.019. - PubMed
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical