Human CD4+CD8α+ Tregs induced by Faecalibacterium prausnitzii protect against intestinal inflammation
- PMID: 35536673
- PMCID: PMC9309064
- DOI: 10.1172/jci.insight.154722
Human CD4+CD8α+ Tregs induced by Faecalibacterium prausnitzii protect against intestinal inflammation
Abstract
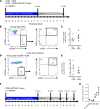
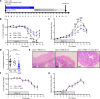
Abundance of Faecalibacterium prausnitzii, a dominant bacterium of the human microbiota that exhibits antiinflammatory effects, is decreased in patients with inflammatory bowel diseases (IBD). In humans, colonic lamina propria contains IL-10-secreting, Foxp3- Tregs characterized by a double expression of CD4 and CD8α (DP8α) and a specificity for F. prausnitzii. This Treg subset is decreased in IBD. The in vivo effect of DP8α cells has not been evaluated yet to our knowledge. Here, using a humanized model of a NSG immunodeficient mouse strain that expresses the HLA D-related allele HLA-DR*0401 but not murine class II (NSG-Ab° DR4) molecules, we demonstrated a protective effect of a HLA-DR*0401-restricted DP8α Treg clone combined with F. prausnitzii administration in a colitis model. In a cohort of patients with IBD, we showed an independent association between the frequency of circulating DP8α cells and disease activity. Finally, we pointed out a positive correlation between F. prausnitzii-specific DP8α Tregs and the amount of F. prausnitzii in fecal microbiota in healthy individuals and patients with ileal Crohn's disease.
Keywords: Gastroenterology; Inflammatory bowel disease; Mouse models; T cells.
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

