Evaluation of Spatially Targeted Scleral Stiffening on Neuroprotection in a Rat Model of Glaucoma
- PMID: 35536721
- PMCID: PMC9100482
- DOI: 10.1167/tvst.11.5.7
Evaluation of Spatially Targeted Scleral Stiffening on Neuroprotection in a Rat Model of Glaucoma
Abstract
Purpose: Scleral stiffening may protect against glaucomatous retinal ganglion cell (RGC) loss or dysfunction associated with ocular hypertension. Here, we assess the potential neuroprotective effects of two treatments designed to stiffen either the entire posterior sclera or only the sclera adjacent to the peripapillary sclera in an experimental model of glaucoma.
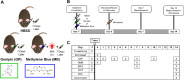
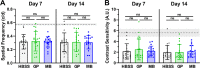
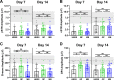
Methods: Rat sclerae were stiffened in vivo using either genipin (crosslinking the entire posterior sclera) or a regionally selective photosensitizer, methylene blue (stiffening only the juxtaperipapillary region surrounding the optic nerve). Ocular hypertension was induced using magnetic microbeads delivered to the anterior chamber. Morphological and functional outcomes, including optic nerve axon count and appearance, retinal thickness measured by optical coherence tomography, optomotor response, and electroretinography traces, were assessed.
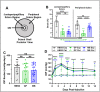
Results: Both local (juxtaperipapillary) and global (whole posterior) scleral stiffening treatments were successful at increasing scleral stiffness, but neither provided demonstrable neuroprotection in hypertensive eyes as assessed by RGC axon counts and appearance, optomotor response, or electroretinography. There was a weak indication that scleral crosslinking protected against retinal thinning as assessed by optical coherence tomography.
Conclusions: Scleral stiffening was not demonstrated to be neuroprotective in ocular hypertensive rats. We hypothesize that the absence of benefit may in part be due to RGC loss associated with the scleral stiffening agents themselves (mild in the case of genipin, and moderate in the case of methylene blue), negating any potential benefit of scleral stiffening.
Translational relevance: The development of scleral stiffening as a neuroprotective treatment will require the identification of better tolerated stiffening protocols and further preclinical testing.
Conflict of interest statement
Disclosure:
Figures


Similar articles
-
Transpupillary collagen photocrosslinking for targeted modulation of ocular biomechanics.Biomaterials. 2021 Apr;271:120735. doi: 10.1016/j.biomaterials.2021.120735. Epub 2021 Feb 24. Biomaterials. 2021. PMID: 33721571 Free PMC article.
-
Assessment of Visual and Retinal Function Following In Vivo Genipin-Induced Scleral Crosslinking.Transl Vis Sci Technol. 2020 Sep 8;9(10):8. doi: 10.1167/tvst.9.10.8. eCollection 2020 Sep. Transl Vis Sci Technol. 2020. PMID: 32974080 Free PMC article.
-
Effects of Peripapillary Scleral Stiffening on the Deformation of the Lamina Cribrosa.Invest Ophthalmol Vis Sci. 2016 May 1;57(6):2666-77. doi: 10.1167/iovs.15-18193. Invest Ophthalmol Vis Sci. 2016. PMID: 27183053 Free PMC article.
-
Altering the way the optic nerve head responds to intraocular pressure-a potential approach to glaucoma therapy.Curr Opin Pharmacol. 2013 Feb;13(1):83-9. doi: 10.1016/j.coph.2012.09.001. Epub 2012 Sep 19. Curr Opin Pharmacol. 2013. PMID: 22999652 Review.
-
Biomechanics of the sclera and effects on intraocular pressure.Int J Ophthalmol. 2016 Dec 18;9(12):1824-1831. doi: 10.18240/ijo.2016.12.21. eCollection 2016. Int J Ophthalmol. 2016. PMID: 28003987 Free PMC article. Review.
Cited by
-
Topical Application of a Collagen Mimetic Peptide Restores Peripapillary Scleral Stiffness Reduced by Ocular Stress.Pharmaceuticals (Basel). 2025 Jun 12;18(6):875. doi: 10.3390/ph18060875. Pharmaceuticals (Basel). 2025. PMID: 40573271 Free PMC article.
-
Three-Dimensional Ultrasound Elastography Detects Age-Related Increase in Anterior Peripapillary Sclera and Optic Nerve Head Compression During IOP Elevation.Invest Ophthalmol Vis Sci. 2023 Jun 1;64(7):16. doi: 10.1167/iovs.64.7.16. Invest Ophthalmol Vis Sci. 2023. PMID: 37289169 Free PMC article.
-
Computational modelling of scleral photocrosslinking: from rat to minipig to human.J R Soc Interface. 2024 Jul;21(216):20240111. doi: 10.1098/rsif.2024.0111. Epub 2024 Jul 31. J R Soc Interface. 2024. PMID: 39081249 Free PMC article.
-
Morphometric Analysis of Retinal Ganglion Cell Axons in Normal and Glaucomatous Brown Norway Rats Optic Nerves.Transl Vis Sci Technol. 2023 Mar 1;12(3):8. doi: 10.1167/tvst.12.3.8. Transl Vis Sci Technol. 2023. PMID: 36917118 Free PMC article.
-
A Comprehensive Protocol for Microbead-Induced Ocular Hypertension in Mice.Methods Mol Biol. 2025;2858:243-264. doi: 10.1007/978-1-0716-4140-8_20. Methods Mol Biol. 2025. PMID: 39433681
References
-
- Quigley HA, Addicks EM, Green WR, Maumenee AE.. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981; 99: 635–649. - PubMed
-
- Weinreb RN, Khaw PT.. Primary open-angle glaucoma. Lancet. 2004; 363: 1711–1720. - PubMed
-
- Klein BE, Klein R, Sponsel WE, et al. .. Prevalence of glaucoma. The Beaver Dam Eye Study. Ophthalmology. 1992; 99: 1499–1504. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

