Imaging articular cartilage in osteoarthritis using targeted peptide radiocontrast agents
- PMID: 35536857
- PMCID: PMC9089912
- DOI: 10.1371/journal.pone.0268223
Imaging articular cartilage in osteoarthritis using targeted peptide radiocontrast agents
Abstract
Background: Established MRI and emerging X-ray contrast agents for non-invasive imaging of articular cartilage rely on non-selective electrostatic interactions with negatively charged proteoglycans. These contrast agents have limited prognostic utility in diseases such as osteoarthritis (OA) due to the characteristic high turnover of proteoglycans. To overcome this limitation, we developed a radiocontrast agent that targets the type II collagen macromolecule in cartilage and used it to monitor disease progression in a murine model of OA.
Methods: To confer radiopacity to cartilage contrast agents, the naturally occurring tyrosine derivative 3,5-diiodo-L-tyrosine (DIT) was introduced into a selective peptide for type II collagen. Synthetic DIT peptide derivatives were synthesised by Fmoc-based solid-phase peptide synthesis and binding to ex vivo mouse tibial cartilage evaluated by high-resolution micro-CT. Di-Iodotyrosinated Peptide Imaging of Cartilage (DIPIC) was performed ex vivo and in vivo 4, 8 and 12 weeks in mice after induction of OA by destabilisation of the medial meniscus (DMM). Finally, human osteochondral plugs were imaged ex vivo using DIPIC.
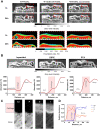
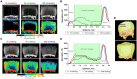
Results: Fifteen DIT peptides were synthesised and tested, yielding seven leads with varying cartilage binding strengths. DIPIC visualised ex vivo murine articular cartilage comparably to the ex vivo contrast agent phosphotungstic acid. Intra-articular injection of contrast agent followed by in vivo DIPIC enabled delineation of damaged murine articular cartilage. Finally, the translational potential of the contrast agent was confirmed by visualisation of ex vivo human cartilage explants.
Conclusion: DIPIC has reduction and refinement implications in OA animal research and potential clinical translation to imaging human disease.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: MMF and NHL are named inventors on patents for radiopaque compounds containing DIT (WO2018020262A1, EP3490614A1). All other authors declare that they have no competing interests.
Figures


Similar articles
-
Synchrotron- and laboratory-based X-ray phase-contrast imaging for imaging mouse articular cartilage in the absence of radiopaque contrast agents.Philos Trans A Math Phys Eng Sci. 2014 Jan 27;372(2010):20130127. doi: 10.1098/rsta.2013.0127. Print 2014 Mar 6. Philos Trans A Math Phys Eng Sci. 2014. PMID: 24470419 Free PMC article.
-
Rapid, automated imaging of mouse articular cartilage by microCT for early detection of osteoarthritis and finite element modelling of joint mechanics.Osteoarthritis Cartilage. 2014 Oct;22(10):1419-28. doi: 10.1016/j.joca.2014.07.014. Osteoarthritis Cartilage. 2014. PMID: 25278053 Free PMC article.
-
Application of autofluorescence robotic histology for quantitative evaluation of the 3-dimensional morphology of murine articular cartilage.Microsc Res Tech. 2017 Dec;80(12):1351-1360. doi: 10.1002/jemt.22948. Epub 2017 Sep 30. Microsc Res Tech. 2017. PMID: 28963813 Free PMC article.
-
Dual-Contrast Agent with Nanoparticle and Molecular Components in Photon-Counting Computed Tomography: Assessing Articular Cartilage Health.Ann Biomed Eng. 2025 Jun;53(6):1423-1438. doi: 10.1007/s10439-025-03715-0. Epub 2025 Mar 28. Ann Biomed Eng. 2025. PMID: 40155520 Free PMC article.
-
Varying development of femoral and tibial subchondral bone tissue and their interaction with articular cartilage during progressing osteoarthritis.Arch Orthop Trauma Surg. 2020 Dec;140(12):1919-1930. doi: 10.1007/s00402-020-03480-w. Epub 2020 May 30. Arch Orthop Trauma Surg. 2020. PMID: 32474697
Cited by
-
Biomechanics of the Human Osteochondral Unit: A Systematic Review.Materials (Basel). 2024 Apr 8;17(7):1698. doi: 10.3390/ma17071698. Materials (Basel). 2024. PMID: 38612211 Free PMC article. Review.
-
Revealing Detailed Cartilage Function Through Nanoparticle Diffusion Imaging: A Computed Tomography & Finite Element Study.Ann Biomed Eng. 2024 Sep;52(9):2584-2595. doi: 10.1007/s10439-024-03552-7. Epub 2024 Jul 16. Ann Biomed Eng. 2024. PMID: 39012563 Free PMC article.
-
Modular iodinated carboxybetaine copolymers as charge-sensitive contrast agents for the detection of cartilage degradation.Mater Today Bio. 2024 Oct 26;29:101302. doi: 10.1016/j.mtbio.2024.101302. eCollection 2024 Dec. Mater Today Bio. 2024. PMID: 39554837 Free PMC article.
References
-
- Joshi NS, Bansal PN, Stewart RC, Snyder BD, Grinstaff MW. Effect of contrast agent charge on visualization of articular cartilage using computed tomography: exploiting electrostatic interactions for improved sensitivity. J Am Chem Soc. 2009;131(37):13234–5. Epub 2009/09/17. doi: 10.1021/ja9053306 . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

