Australia as a global sink for the genetic diversity of avian influenza A virus
- PMID: 35536868
- PMCID: PMC9089890
- DOI: 10.1371/journal.ppat.1010150
Australia as a global sink for the genetic diversity of avian influenza A virus
Abstract
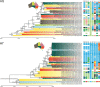
Most of our understanding of the ecology and evolution of avian influenza A virus (AIV) in wild birds is derived from studies conducted in the northern hemisphere on waterfowl, with a substantial bias towards dabbling ducks. However, relevant environmental conditions and patterns of avian migration and reproduction are substantially different in the southern hemisphere. Through the sequencing and analysis of 333 unique AIV genomes collected from wild birds collected over 15 years we show that Australia is a global sink for AIV diversity and not integrally linked with the Eurasian gene pool. Rather, AIV are infrequently introduced to Australia, followed by decades of isolated circulation and eventual extinction. The number of co-circulating viral lineages varies per subtype. AIV haemagglutinin (HA) subtypes that are rarely identified at duck-centric study sites (H8-12) had more detected introductions and contemporary co-circulating lineages in Australia. Combined with a lack of duck migration beyond the Australian-Papuan region, these findings suggest introductions by long-distance migratory shorebirds. In addition, on the available data we found no evidence of directional or consistent patterns in virus movement across the Australian continent. This feature corresponds to patterns of bird movement, whereby waterfowl have nomadic and erratic rainfall-dependant distributions rather than consistent intra-continental migratory routes. Finally, we detected high levels of virus gene segment reassortment, with a high diversity of AIV genome constellations across years and locations. These data, in addition to those from other studies in Africa and South America, clearly show that patterns of AIV dynamics in the Southern Hemisphere are distinct from those in the temperate north.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

