What Drives Radical Halogenation versus Hydroxylation in Mononuclear Nonheme Iron Complexes? A Combined Experimental and Computational Study
- PMID: 35537044
- PMCID: PMC9228086
- DOI: 10.1021/jacs.2c01375
What Drives Radical Halogenation versus Hydroxylation in Mononuclear Nonheme Iron Complexes? A Combined Experimental and Computational Study
Abstract
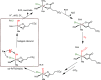
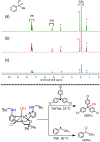
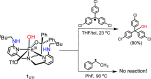
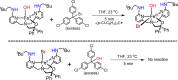
Nonheme iron halogenases are unique enzymes in nature that selectively activate an aliphatic C-H bond of a substrate to convert it into C-X (X = Cl/Br, but not F/I). It is proposed that they generate an FeIII(OH)(X) intermediate in their catalytic cycle. The analogous FeIII(OH) intermediate in nonheme iron hydroxylases transfers OH• to give alcohol product, whereas the halogenases transfer X• to the carbon radical substrate. There remains significant debate regarding what factors control their remarkable selectivity of the halogenases. The reactivity of the complexes FeIII(BNPAPh2O)(OH)(X) (X = Cl, Br) with a secondary carbon radical (R•) is described. It is found that X• transfer occurs with a secondary carbon radical, as opposed to OH• transfer with tertiary radicals. Comprehensive computational studies involving density functional theory were carried out to examine the possible origins of this selectivity. The calculations reproduce the experimental findings, which indicate that halogen transfer is not observed for the tertiary radicals because of a nonproductive equilibrium that results from the endergonic nature of these reactions, despite a potentially lower reaction barrier for the halogenation pathway. In contrast, halogen transfer is favored for secondary carbon radicals, for which the halogenated product complex is thermodynamically more stable than the reactant complex. These results are rationalized by considering the relative strengths of the C-X bonds that are formed for tertiary versus secondary carbon centers. The computational analysis also shows that the reaction barrier for halogen transfer is significantly dependent on secondary coordination sphere effects, including steric and H-bonding interactions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

