New intranasal and injectable gene therapy for healthy life extension
- PMID: 35537048
- PMCID: PMC9171804
- DOI: 10.1073/pnas.2121499119
New intranasal and injectable gene therapy for healthy life extension
Erratum in
-
Correction for Jaijyan et al., New intranasal and injectable gene therapy for healthy life extension.Proc Natl Acad Sci U S A. 2022 Aug 30;119(35):e2212836119. doi: 10.1073/pnas.2212836119. Epub 2022 Aug 24. Proc Natl Acad Sci U S A. 2022. PMID: 36001694 Free PMC article. No abstract available.
-
Correction for Jaijyan et al., New intranasal and injectable gene therapy for healthy life extension.Proc Natl Acad Sci U S A. 2023 Aug 8;120(32):e2311483120. doi: 10.1073/pnas.2311483120. Epub 2023 Jul 31. Proc Natl Acad Sci U S A. 2023. PMID: 37523572 Free PMC article. No abstract available.
Retraction in
-
Retraction for Jaijyan et al., New intranasal and injectable gene therapy for healthy life extension.Proc Natl Acad Sci U S A. 2025 Aug 19;122(33):e2519570122. doi: 10.1073/pnas.2519570122. Epub 2025 Aug 11. Proc Natl Acad Sci U S A. 2025. PMID: 40789040 Free PMC article. No abstract available.
Abstract
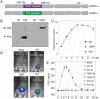
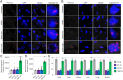
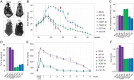
As the global elderly population grows, it is socioeconomically and medically critical to provide diverse and effective means of mitigating the impact of aging on human health. Previous studies showed that the adeno-associated virus (AAV) vector induced overexpression of certain proteins, which can suppress or reverse the effects of aging in animal models. In our study, we sought to determine whether the high-capacity cytomegalovirus vector (CMV) can be an effective and safe gene delivery method for two such protective factors: telomerase reverse transcriptase (TERT) and follistatin (FST). We found that the mouse cytomegalovirus (MCMV) carrying exogenous TERT or FST (MCMVTERT or MCMVFST) extended median lifespan by 41.4% and 32.5%, respectively. We report CMV being used successfully as both an intranasal and injectable gene therapy system to extend longevity. Specifically, this treatment significantly improved glucose tolerance, physical performance, as well as preventing body mass loss and alopecia. Further, telomere shortening associated with aging was ameliorated by TERT and mitochondrial structure deterioration was halted in both treatments. Intranasal and injectable preparations performed equally well in safely and efficiently delivering gene therapy to multiple organs, with long-lasting benefits and without carcinogenicity or unwanted side effects. Translating this research to humans could have significant benefits associated with quality of life and an increased health span.
Keywords: TERT; aging; cytomegalovirus; follistatin; gene therapy.
Conflict of interest statement
Competing interest statement: D.K., and E.L.P. are employees of BioViva, Inc. BioViva owns the patent pending technology on the research herein. E.L.P. and D.K. manage and sit on the board of directors of BioViva USA, Inc. G.C. is a member of the advisory board for and a shareholder in BioViva USA, Inc. He is not an inventor on the patents.
Figures


References
-
- Weinrich S. L., et al. , Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nat. Genet. 17, 498–502 (1997). - PubMed
-
- Bodnar A. G., et al. , Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349–352 (1998). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

