Striatal mechanism of the restless legs syndrome
- PMID: 35537196
- PMCID: PMC9272194
- DOI: 10.1093/sleep/zsac110
Striatal mechanism of the restless legs syndrome
Abstract
Study objectives: Brain iron deficiency has been reported to be associated with the restless legs syndrome (RLS). However, 30%-50% of RLS patients do not respond to iron therapy, indicating that mechanisms other than brain iron deficiency may also participate in this disease. The striatum is known to be involved in the modulation of motor activity. We speculated that dysfunction of the striatum may induce RLS.
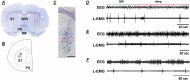
Methods: Two groups, wild-type (WT) and iron-deficient (ID) rats were used. Each group was divided into two subgroups, control and N-methyl-d-aspartate striatal-lesioned. After baseline recording, striatal-lesioned wild-type (WT-STL) and striatal-lesioned iron-deficient (ID-STL) rats were given pramipexole and thioperamide injections. Iron-deficient and ID-STL rats were then given a standard rodent diet for 4 weeks, and their sleep and motor activity were recorded.
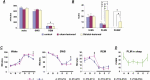
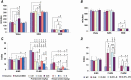
Results: WT-STL rats showed periodic leg movements (PLM) in wake, an increase in PLM in slow wave sleep (SWS), a decrease in rapid-eye-movement sleep, and a decrease in the daily average duration of episodes in SWS. The sleep-wake pattern and motor activity did not differ between ID and ID-STL rats. Thioperamide or pramipexole injection decreased PLM in sleep and in wake in WT-STL rats and ID-STL rats. Unlike ID rats, whose motor hyperactivity can be reversed by iron replacement, PLM in wake and in sleep in ID-STL rats were not fully corrected by iron treatment.
Conclusions: Lesions of the striatum generate RLS-like activity in rats. Dysfunction of the striatum may be responsible for failure to respond to iron treatment in some human RLS patients.
Keywords: iron deficient; iron therapy; neurotoxic lesions; periodic leg movement; pramipexole; thioperamide.
© The Author(s) 2022. Published by Oxford University Press on behalf of Sleep Research Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Joo EY, et al. Reduced cerebral blood perfusion of putamen and insular cortex in patients with idiopathic restless legs syndrome. J Korea Sleep Res Soc. 2012;9(1):10–14. doi: 10.13078/jksrs.12003. - DOI

