Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity
- PMID: 35537412
- PMCID: PMC9221568
- DOI: 10.1016/j.ccell.2022.04.004
Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity
Abstract
Immune checkpoint blockade (ICB) therapy frequently induces immune-related adverse events. To elucidate the underlying immunobiology, we performed a deep immune analysis of intestinal, colitis, and tumor tissue from ICB-treated patients with parallel studies in preclinical models. Expression of interleukin-6 (IL-6), neutrophil, and chemotactic markers was higher in colitis than in normal intestinal tissue; T helper 17 (Th17) cells were more prevalent in immune-related enterocolitis (irEC) than T helper 1 (Th1). Anti-cytotoxic T-lymphocyte-associated antigen 4 (anti-CTLA-4) induced stronger Th17 memory in colitis than anti-program death 1 (anti-PD-1). In murine models, IL-6 blockade associated with improved tumor control and a higher density of CD4+/CD8+ effector T cells, with reduced Th17, macrophages, and myeloid cells. In an experimental autoimmune encephalomyelitis (EAE) model with tumors, combined IL-6 blockade and ICB enhanced tumor rejection while simultaneously mitigating EAE symptoms versus ICB alone. IL-6 blockade with ICB could de-couple autoimmunity from antitumor immunity.
Trial registration: ClinicalTrials.gov NCT04940299.
Keywords: EAE; TC1/TC17; Th1/Th17; Th17 memory; arthritis; colitis; immune checkpoint blockade; immunity; interleukin-6; melanoma; toxicity.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.D. received Institution Research funds: Bristol Myers and Squibb, Merck, Pfizer, Nektar Therapeutics, Idera Pharmaceuticals, Apexigen and advisory board fees: Bristol Myers and Squibb, Nektar Therapeutics, Idera Pharmaceuticals, Iovance Therapeutics, Apexigen. M.A.D. has been a consultant to Roche/Genentech, Array, Pfizer, Novartis, BMS, GSK, Sanofi-Aventis, Vaccinex, Apexigen, Eisai, Iovance, and ABM Therapeutics, and he has been the PI of research grants to MD Anderson by Roche/Genentech, GSK, Sanofi-Aventis, Merck, Myriad, Oncothyreon, and ABM Therapeutics.
Figures
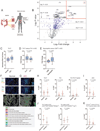
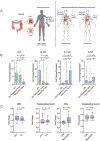



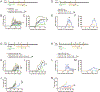
Comment in
-
IL-6 blockade in cancer patients treated with immune checkpoint blockade: A win-win strategy.Cancer Cell. 2022 May 9;40(5):450-451. doi: 10.1016/j.ccell.2022.04.010. Epub 2022 May 9. Cancer Cell. 2022. PMID: 35537409
References
-
- Abu-Sbeih H, Ali FS, Naqash AR, Owen DH, Patel S, Otterson GA, Kendra K, Ricciuti B, Chiari R, De Giglio A, et al. (2019). Resumption of Immune Checkpoint Inhibitor Therapy After Immune-Mediated Colitis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 37, 2738–2745. - PMC - PubMed
-
- Bamias G, Delladetsima I, Perdiki M, Siakavellas SI, Goukos D, Papatheodoridis GV, Daikos GL, and Gogas H (2017). Immunological Characteristics of Colitis Associated with Anti-CTLA-4 Antibody Therapy. Cancer investigation 35, 443–455. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

