Advancing Pharmacogenomics from Single-Gene to Preemptive Testing
- PMID: 35537468
- PMCID: PMC9483991
- DOI: 10.1146/annurev-genom-111621-102737
Advancing Pharmacogenomics from Single-Gene to Preemptive Testing
Abstract
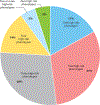
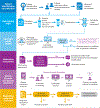
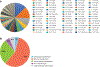
Pharmacogenomic testing can be an effective tool to enhance medication safety and efficacy. Pharmacogenomically actionable medications are widely used, and approximately 90-95% of individuals have an actionable genotype for at least one pharmacogene. For pharmacogenomic testing to have the greatest impact on medication safety and clinical care, genetic information should be made available at the time of prescribing (preemptive testing). However, the use of preemptive pharmacogenomic testing is associated with some logistical concerns, such as consistent reimbursement, processes for reporting preemptive results over an individual's lifetime, and result portability. Lessons can be learned from institutions that have implemented preemptive pharmacogenomic testing. In this review, we discuss the rationale and best practices for implementing pharmacogenomics preemptively.
Keywords: clinical decision support; genomic medicine; individualized medicine; personalized medicine; pharmacogenetics; pharmacogenomics; precision medicine.
Figures

Similar articles
-
Preemptive Pharmacogenomic Testing for Precision Medicine: A Comprehensive Analysis of Five Actionable Pharmacogenomic Genes Using Next-Generation DNA Sequencing and a Customized CYP2D6 Genotyping Cascade.J Mol Diagn. 2016 May;18(3):438-445. doi: 10.1016/j.jmoldx.2016.01.003. Epub 2016 Mar 3. J Mol Diagn. 2016. PMID: 26947514 Free PMC article.
-
Clinically actionable genotypes for anticancer prescribing among >1500 patients with pharmacogenomic testing.Cancer. 2022 Apr 15;128(8):1649-1657. doi: 10.1002/cncr.34104. Epub 2022 Jan 28. Cancer. 2022. PMID: 35090043 Free PMC article.
-
Adoption of a clinical pharmacogenomics implementation program during outpatient care--initial results of the University of Chicago "1,200 Patients Project".Am J Med Genet C Semin Med Genet. 2014 Mar;166C(1):68-75. doi: 10.1002/ajmg.c.31385. Epub 2014 Mar 10. Am J Med Genet C Semin Med Genet. 2014. PMID: 24616296 Free PMC article.
-
Pharmacogenomics Implementation: Considerations for Selecting a Reference Laboratory.Pharmacotherapy. 2017 Sep;37(9):1014-1022. doi: 10.1002/phar.1985. Epub 2017 Sep 3. Pharmacotherapy. 2017. PMID: 28699700 Review.
-
The challenges of implementing pharmacogenomic testing in the clinic.Expert Rev Pharmacoecon Outcomes Res. 2017 Dec;17(6):567-577. doi: 10.1080/14737167.2017.1385395. Epub 2017 Oct 3. Expert Rev Pharmacoecon Outcomes Res. 2017. PMID: 28949250 Review.
Cited by
-
Clinician adherence to pharmacogenomics prescribing recommendations in clinical decision support alerts.J Am Med Inform Assoc. 2022 Dec 13;30(1):132-138. doi: 10.1093/jamia/ocac187. J Am Med Inform Assoc. 2022. PMID: 36228116 Free PMC article.
-
Pharmacogenomics in the UK National Health Service: Progress towards implementation.Br J Clin Pharmacol. 2025 Aug;91(8):2241-2250. doi: 10.1002/bcp.70109. Epub 2025 Jun 3. Br J Clin Pharmacol. 2025. PMID: 40460990 Free PMC article. Review.
-
A feasibility study on implementing pre-emptive pharmacogenomics testing in outpatient clinics in Singapore (IMPT study).Pharmacogenomics J. 2025 Mar 12;25(1-2):7. doi: 10.1038/s41397-025-00366-1. Pharmacogenomics J. 2025. PMID: 40074758 Free PMC article.
-
Pharmacogenomics in practice: a review and implementation guide.Front Pharmacol. 2023 May 18;14:1189976. doi: 10.3389/fphar.2023.1189976. eCollection 2023. Front Pharmacol. 2023. PMID: 37274118 Free PMC article. Review.
-
Real-World Utilization of Medications With Pharmacogenetic Recommendations in Older Adults: A Scoping Review.Clin Transl Sci. 2025 Feb;18(2):e70126. doi: 10.1111/cts.70126. Clin Transl Sci. 2025. PMID: 39967300 Free PMC article.
References
-
- Am. Soc. Health-Syst. Pharm. 2018. Required competency areas, goals and objectives for postgraduate year two (PGY2) clinical pharmacogenomics pharmacy residencies. Resid. Program Doc., Am. Soc. Health-Syst. Pharm, Bethesda, MD. https://www.ashp.org/-/media/assets/professional-development/residencies...
-
- Am. Soc. Health-Syst. Pharm. 2021. Residency directory. American Society of Health-System Pharmacists. https://accreditation.ashp.org/directory/#/program/residency
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

