Advancing Pharmacogenomics from Single-Gene to Preemptive Testing
- PMID: 35537468
- PMCID: PMC9483991
- DOI: 10.1146/annurev-genom-111621-102737
Advancing Pharmacogenomics from Single-Gene to Preemptive Testing
Abstract
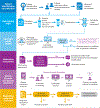
Pharmacogenomic testing can be an effective tool to enhance medication safety and efficacy. Pharmacogenomically actionable medications are widely used, and approximately 90-95% of individuals have an actionable genotype for at least one pharmacogene. For pharmacogenomic testing to have the greatest impact on medication safety and clinical care, genetic information should be made available at the time of prescribing (preemptive testing). However, the use of preemptive pharmacogenomic testing is associated with some logistical concerns, such as consistent reimbursement, processes for reporting preemptive results over an individual's lifetime, and result portability. Lessons can be learned from institutions that have implemented preemptive pharmacogenomic testing. In this review, we discuss the rationale and best practices for implementing pharmacogenomics preemptively.
Keywords: clinical decision support; genomic medicine; individualized medicine; personalized medicine; pharmacogenetics; pharmacogenomics; precision medicine.
Figures

References
-
- Am. Soc. Health-Syst. Pharm. 2018. Required competency areas, goals and objectives for postgraduate year two (PGY2) clinical pharmacogenomics pharmacy residencies. Resid. Program Doc., Am. Soc. Health-Syst. Pharm, Bethesda, MD. https://www.ashp.org/-/media/assets/professional-development/residencies...
-
- Am. Soc. Health-Syst. Pharm. 2021. Residency directory. American Society of Health-System Pharmacists. https://accreditation.ashp.org/directory/#/program/residency
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

