Modeling transmission of SARS-CoV-2 Omicron in China
- PMID: 35537471
- PMCID: PMC9307473
- DOI: 10.1038/s41591-022-01855-7
Modeling transmission of SARS-CoV-2 Omicron in China
Abstract
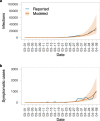
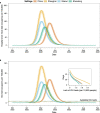
Having adopted a dynamic zero-COVID strategy to respond to SARS-CoV-2 variants with higher transmissibility since August 2021, China is now considering whether, and for how long, this policy can remain in place. The debate has thus shifted towards the identification of mitigation strategies for minimizing disruption to the healthcare system in the case of a nationwide epidemic. To this aim, we developed an age-structured stochastic compartmental susceptible-latent-infectious-removed-susceptible model of SARS-CoV-2 transmission calibrated on the initial growth phase for the 2022 Omicron outbreak in Shanghai, to project COVID-19 burden (that is, number of cases, patients requiring hospitalization and intensive care, and deaths) under hypothetical mitigation scenarios. The model also considers age-specific vaccine coverage data, vaccine efficacy against different clinical endpoints, waning of immunity, different antiviral therapies and nonpharmaceutical interventions. We find that the level of immunity induced by the March 2022 vaccination campaign would be insufficient to prevent an Omicron wave that would result in exceeding critical care capacity with a projected intensive care unit peak demand of 15.6 times the existing capacity and causing approximately 1.55 million deaths. However, we also estimate that protecting vulnerable individuals by ensuring accessibility to vaccines and antiviral therapies, and maintaining implementation of nonpharmaceutical interventions could be sufficient to prevent overwhelming the healthcare system, suggesting that these factors should be points of emphasis in future mitigation policies.
© 2022. The Author(s).
Conflict of interest statement
H.Y. received research funding from Sanofi Pasteur, GlaxoSmithKline, Yichang HEC Changjiang Pharmaceutical Company, Shanghai Roche Pharmaceutical Company and SINOVAC Biotech Ltd. Except for research funding from SINOVAC Biotech Ltd, which is related to the data analysis of clinical trials of immunogenicity and safety of CoronaVac, the others are not related to COVID-19. M.A. has received research funding from Seqirus; the funding is not related to COVID-19. All the other authors have no competing interests.
Figures




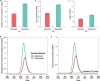
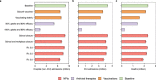
Comment in
-
Can China avoid a wave of deaths if it lifts strict zero COVID policy?Nature. 2022 Dec;612(7939):203. doi: 10.1038/d41586-022-04235-w. Nature. 2022. PMID: 36450962 No abstract available.
References
-
- Tracking of VOC Omicron. Global Initiative of Sharing All Influenza Data (GISAID) (29 January 2022); https://www.gisaid.org/hcov19-variants/
-
- Lyngse, F.P. SARS-CoV-2 Omicron VOC transmission in Danish households. Preprint at medRXiv10.1101/2021.12.27.21268278 (2021).
-
- Pearson, C.A.B. et al. Bounding the levels of transmissibility & immune evasion of the Omicron variant in South Africa. Preprint at medRxiv10.1101/2021.12.19.21268038 (2021).
-
- Gozzi, N. et al. Preliminary modeling estimates of the relative transmissibility and immune escape of the Omicron SARS-CoV-2 variant of concern in South Africa. Preprint at medRxiv10.1101/2022.01.04.22268721 (2022).
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

