Pathway-specific inhibition of critical projections from the mediodorsal thalamus to the frontal cortex controls kindled seizures
- PMID: 35537572
- PMCID: PMC9361177
- DOI: 10.1016/j.pneurobio.2022.102286
Pathway-specific inhibition of critical projections from the mediodorsal thalamus to the frontal cortex controls kindled seizures
Abstract
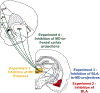
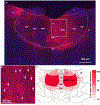
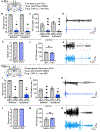
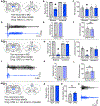
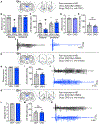
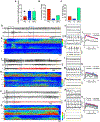
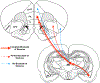
There is a large unmet need for improved treatment for temporal lobe epilepsy (TLE); circuit-specific manipulation that disrupts the initiation and propagation of seizures is promising in this regard. The midline thalamus, including the mediodorsal nucleus (MD) is a critical distributor of seizure activity, but its afferent and efferent pathways that mediate seizure activity are unknown. Here, we used chemogenetics to silence input and output projections of the MD to discrete regions of the frontal cortex in the kindling model of TLE in rats. Chemogenetic inhibition of the projection from the amygdala to the MD abolished seizures, an effect that was replicated using optogenetic inhibition. Chemogenetic inhibition of projections from the MD to the prelimbic cortex likewise abolished seizures. By contrast, inhibition of projections from the MD to other frontal regions produced partial (orbitofrontal cortex, infralimbic cortex) or no (cingulate, insular cortex) attenuation of behavioral or electrographic seizure activity. These results highlight the particular importance of projections from MD to prelimbic cortex in the propagation of amygdala-kindled seizures.
Keywords: Amygdala; Chemogenetics; Deep brain stimulation; Epilepsy; Frontal cortex; Hippocampus; Kindling; Optogenetics; Temporal lobe epilepsy; Thalamus.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Figures




References
-
- Alcaraz F, Marchand AR, Courtand G, Coutureau E, & Wolff M (2016). Parallel inputs from the mediodorsal thalamus to the prefrontal cortex in the rat. The European Journal of Neuroscience, 44(3), 1972–1986. - PubMed
-
- Arikan R, Blake NMJ, Erinjeri JP, Woolsey TA, Giraud L, & Highstein SM (2002). A method to measure the effective spread of focally injected muscimol into the central nervous system with electrophysiology and light microscopy. Journal of Neuroscience Methods, 118(1), 51–57. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

