Agreement of treatment effects from observational studies and randomized controlled trials evaluating hydroxychloroquine, lopinavir-ritonavir, or dexamethasone for covid-19: meta-epidemiological study
- PMID: 35537738
- PMCID: PMC9086409
- DOI: 10.1136/bmj-2021-069400
Agreement of treatment effects from observational studies and randomized controlled trials evaluating hydroxychloroquine, lopinavir-ritonavir, or dexamethasone for covid-19: meta-epidemiological study
Abstract
Objective: To systematically identify, match, and compare treatment effects and study demographics from individual or meta-analysed observational studies and randomized controlled trials (RCTs) evaluating the same covid-19 treatments, comparators, and outcomes.
Design: Meta-epidemiological study.
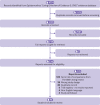
Data sources: National Institutes of Health Covid-19 Treatment Guidelines, a living review and network meta-analysis published in The BMJ, a living systematic review with meta-analysis and trial sequential analysis in PLOS Medicine (The LIVING Project), and the Epistemonikos "Living OVerview of Evidence" (L·OVE) evidence database.
Eligibility criteria for selection of studies: RCTs in The BMJ's living review that directly compared any of the three most frequently studied therapeutic interventions for covid-19 across all data sources (that is, hydroxychloroquine, lopinavir-ritonavir, or dexamethasone) for any safety and efficacy outcomes. Observational studies that evaluated the same interventions, comparisons, and outcomes that were reported in The BMJ's living review.
Data extraction and synthesis: Safety and efficacy outcomes from observational studies were identified and treatment effects for dichotomous (odds ratios) or continuous (mean differences or ratios of means) outcomes were calculated and, when possible, meta-analyzed to match the treatment effects from individual RCTs or meta-analyses of RCTs reported in The BMJ's living review with the same interventions, comparisons, and outcomes (that is, matched pairs). The analysis compared the distribution of study demographics and the agreement between treatment effects from matched pairs. Matched pairs were in agreement if both observational and RCT treatment effects were significantly increasing or decreasing (P<0.05) or if both treatment effects were not significant (P≥0.05).
Results: 17 new, independent meta-analyses of observational studies were conducted that compared hydroxychloroquine, lopinavir-ritonavir, or dexamethasone with an active or placebo comparator for any safety or efficacy outcomes in covid-19 treatment. These studies were matched and compared with 17 meta-analyses of RCTs reported in The BMJ's living review. 10 additional matched pairs with only one observational study and/or one RCT were identified. Across all 27 matched pairs, 22 had adequate reporting of demographical and clinical data for all individual studies. All 22 matched pairs had studies with overlapping distributions of sex, age, and disease severity. Overall, 21 (78%) of the 27 matched pairs had treatment effects that were in agreement. Among the 17 matched pairs consisting of meta-analyses of observational studies and meta-analyses of RCTs, 14 (82%) were in agreement; seven (70%) of the 10 matched pairs consisting of at least one observational study or one RCT were in agreement. The 18 matched pairs with treatment effects for dichotomous outcomes had a higher proportion of agreement (n=16, 89%) than did the nine matched pairs with treatment effects for continuous outcomes (n=5, 56%).
Conclusions: Meta-analyses of observational studies and RCTs evaluating treatments for covid-19 have summary treatment effects that are generally in agreement. Although our evaluation is limited to three covid-19 treatments, these findings suggest that meta-analyzed evidence from observational studies might complement, but should not replace, evidence collected from RCTs.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health for the submitted work; JSR is the US outreach and associate research editor at The BMJ and currently receives research support through Yale University from Johnson & Johnson to develop methods of clinical trial data sharing, from the Medical Device Innovation Consortium as part of the National Evaluation System for Health Technology, from the US Food and Drug Administration for the Yale-Mayo Clinic Center for Excellence in Regulatory Science and Innovation program (U01FD005938), from the Agency for Healthcare Research and Quality (R01HS022882), from the National Heart, Lung and Blood Institute of the National Institutes of Health (R01HS025164, R01HL144644), and from the Laura and John Arnold Foundation to establish the Good Pharma Scorecard at Bioethics International; JSR is also an expert witness at the request of Relator’s attorneys, the Greene law firm, in a lawsuit alleging violations of the False Claims Act and Anti-Kickback Statute against Biogen; JDW currently received research support from the US Food and Drug Administration and through Yale University from Johnson & Johnson to develop methods of clinical trial data sharing.
Figures




Similar articles
-
Effectiveness of Interferon Beta 1a, compared to Interferon Beta 1b and the usual therapeutic regimen to treat adults with moderate to severe COVID-19: structured summary of a study protocol for a randomized controlled trial.Trials. 2020 Jun 3;21(1):473. doi: 10.1186/s13063-020-04382-3. Trials. 2020. PMID: 32493468 Free PMC article.
-
Safety and efficacy of antiviral combination therapy in symptomatic patients of Covid-19 infection - a randomised controlled trial (SEV-COVID Trial): A structured summary of a study protocol for a randomized controlled trial.Trials. 2020 Oct 20;21(1):866. doi: 10.1186/s13063-020-04774-5. Trials. 2020. PMID: 33081849 Free PMC article. Clinical Trial.
-
SARS-CoV-2 pharmacologic therapies and their safety/effectiveness according to level of evidence.Am J Emerg Med. 2020 Nov;38(11):2405-2415. doi: 10.1016/j.ajem.2020.08.091. Epub 2020 Sep 1. Am J Emerg Med. 2020. PMID: 33041111 Free PMC article. Review.
-
Repurposing of drugs for COVID-19: a systematic review and meta-analysis.Panminerva Med. 2022 Mar;64(1):96-114. doi: 10.23736/S0031-0808.20.04024-0. Epub 2020 Oct 19. Panminerva Med. 2022. PMID: 33073552
-
QTc prolongation in COVID-19 patients treated with hydroxychloroquine, chloroquine, azithromycin, or lopinavir/ritonavir: A systematic review and meta-analysis.Pharmacoepidemiol Drug Saf. 2021 Jun;30(6):694-706. doi: 10.1002/pds.5234. Epub 2021 Apr 3. Pharmacoepidemiol Drug Saf. 2021. PMID: 33772933 Free PMC article.
Cited by
-
Totality of evidence of the effectiveness of repurposed therapies for COVID-19: Can we use real-world studies alongside randomized controlled trials?Clin Transl Sci. 2023 Oct;16(10):1842-1855. doi: 10.1111/cts.13591. Epub 2023 Aug 28. Clin Transl Sci. 2023. PMID: 37466279 Free PMC article.
-
Evaluating the impact of including non-randomised studies of interventions in meta-analysis of randomised controlled trials: a protocol for a meta-epidemiological study.BMJ Open. 2023 Jul 26;13(7):e073232. doi: 10.1136/bmjopen-2023-073232. BMJ Open. 2023. PMID: 37495391 Free PMC article. Clinical Trial.
-
Preoperative psychological health impacts pain and disability outcomes following anterior cervical discectomy and fusion for cervical radiculopathy.Sci Rep. 2025 Apr 28;15(1):14861. doi: 10.1038/s41598-025-97575-2. Sci Rep. 2025. PMID: 40295568 Free PMC article.
-
Impact of mass media campaigns on knowledge of malaria prevention measures among pregnant mothers in Uganda: a propensity score-matched analysis.Malar J. 2024 Aug 24;23(1):256. doi: 10.1186/s12936-024-05083-x. Malar J. 2024. PMID: 39182108 Free PMC article.
-
Design differences and variation in results between randomised trials and non-randomised emulations: meta-analysis of RCT-DUPLICATE data.BMJ Med. 2024 Feb 5;3(1):e000709. doi: 10.1136/bmjmed-2023-000709. eCollection 2024. BMJ Med. 2024. PMID: 38348308 Free PMC article.
References
-
- NEJM. Real-world evidence—what is it and what can it tell us? Accessed 23 December, 2020. https://www.nejm.org/doi/pdf/10.1056/NEJMsb1609216 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
