Myeloid CCR2 Promotes Atherosclerosis after AKI
- PMID: 35537780
- PMCID: PMC9342642
- DOI: 10.1681/ASN.2022010048
Myeloid CCR2 Promotes Atherosclerosis after AKI
Abstract
Background: The risk of cardiovascular events rises after AKI. Leukocytes promote atherosclerotic plaque growth and instability. We established a model of enhanced remote atherosclerosis after renal ischemia-reperfusion (IR) injury and investigated the underlying inflammatory mechanisms.
Methods: Atherosclerotic lesions and inflammation were investigated in native and bone marrow-transplanted LDL receptor-deficient (LDLr-/- ) mice after unilateral renal IR injury using histology, flow cytometry, and gene expression analysis.
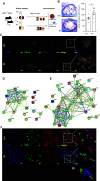
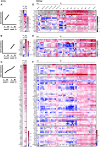
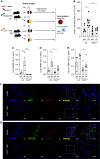
Results: Aortic root atherosclerotic lesions were significantly larger after renal IR injury than in controls. A gene expression screen revealed enrichment for chemokines and their cognate receptors in aortas of IR-injured mice in early atherosclerosis, and of T cell-associated genes in advanced disease. Confocal microscopy revealed increased aortic macrophage proximity to T cells. Differential aortic inflammatory gene regulation in IR-injured mice largely paralleled the pattern in the injured kidney. Single-cell analysis identified renal cell types that produced soluble mediators upregulated in the atherosclerotic aorta. The analysis revealed a marked early increase in Ccl2, which CCR2+ myeloid cells mainly expressed. CCR2 mediated myeloid cell homing to the post-ischemic kidney in a cell-individual manner. Reconstitution with Ccr2-/- bone marrow dampened renal post-ischemic inflammation, reduced aortic Ccl2 and inflammatory macrophage marker CD11c, and abrogated excess aortic atherosclerotic plaque formation after renal IR.
Conclusions: Our data introduce an experimental model of remote proatherogenic effects of renal IR and delineate myeloid CCR2 signaling as a mechanistic requirement. Monocytes should be considered as mobile mediators when addressing systemic vascular sequelae of kidney injury.
Keywords: acute kidney injury; arteriosclerosis; cardiovascular disease; chronic inflammation; macrophages; renal ischemia.
Copyright © 2022 by the American Society of Nephrology.
Figures




References
-
- Shiao C-C, Wu P-C, Huang T-M, Lai T-S, Yang W-S, Wu C-H, et al. ; National Taiwan University Hospital Study Group on Acute Renal Failure (NSARF) and the Taiwan Consortium for Acute Kidney Injury and Renal Diseases (CAKs) : Long-term remote organ consequences following acute kidney injury. Crit Care 19: 438, 2015 - PMC - PubMed
-
- Doi K, Rabb H: Impact of acute kidney injury on distant organ function: Recent findings and potential therapeutic targets. Kidney Int 89: 555–564, 2016 - PubMed
-
- Saratzis A, Harrison S, Barratt J, Sayers RD, Sarafidis PA, Bown MJ: Intervention associated acute kidney injury and long-term cardiovascular outcomes. Am J Nephrol 42: 285–294, 2015 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

