Potential pharmacologic interventions targeting TLR signaling in placental malaria
- PMID: 35537977
- PMCID: PMC7614649
- DOI: 10.1016/j.pt.2022.04.002
Potential pharmacologic interventions targeting TLR signaling in placental malaria
Abstract
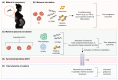
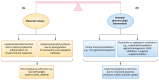
Complications from placental malaria cause poor pregnancy outcomes, including low birthweight, preterm delivery, and stillbirths. Many of these complications are driven by maternal innate proinflammatory responses to the sequestration of Plasmodium falciparum in the placenta. However, recent studies show that, in reaction to maternal innate immune responses that are detrimental to the fetus, the fetus mounts innate immune counter-responses that ameliorate pregnancy outcomes. Such fetal-maternal conflict in placental malaria has potential for pharmacologic modulation for better pregnancy outcomes. Here, we discuss placental malaria pathogenesis, its complications, and the role of innate immunity and fetal-maternal innate immune conflict in placental malaria. Finally, we discuss pharmacologic immunomodulatory strategies and agents with the potential to improve placental malaria outcomes.
Keywords: fetal–maternal immune conflict; innate immune responses; placental malaria; pregnancy outcomes.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
References
-
- World Health Organization. World malaria report 2021. 2021. [Accessed: 08-Mar-2022]. [Online].Available: https://www.who.int/teams/global-malaria-programme/reports/world-malaria....
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

