CD82 palmitoylation site mutations at Cys5+Cys74 affect EGFR internalization and metabolism through recycling pathway
- PMID: 35538033
- PMCID: PMC9828285
- DOI: 10.3724/abbs.2022011
CD82 palmitoylation site mutations at Cys5+Cys74 affect EGFR internalization and metabolism through recycling pathway
Abstract
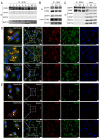
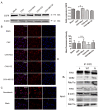
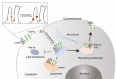
Tetraspanin CD82 often participates in regulating the function of epidermal growth factor receptor (EGFR) and hepatocyte growth factor receptor (c-Met). Palmitoylation is a post-translational modification that contributes to tetraspanin web formation and affects tetraspanin-dependent cell signaling. However, the molecular mechanisms by which CD82 palmitoylation affects the localization and stability of EGFR and c-Met have not yet been elucidated. This study focuses on the expression and distribution of EGFR and c-Met in breast cancer as well as the related metabolic pathways and molecular mechanisms associated with different CD82 palmitoylation site mutations. The results show that CD82 with a palmitoylation mutation at Cys5+Cys74 can promote the internalization of EGFR. EGFR is internalized and strengthened by direct binding to CD82 with the tubulin assistance and located at the recycling endosome. After studying the recycling pathway marker proteins Rab11a and FIP2, we found that formation of the EGFR/CD82/Rab11a/FIP2 complex promotes the internalization and metabolism of EGFR through the recycling pathway and results in the re-expression of EGFR and CD82 on the cell membrane.
Keywords: CD82; EGFR; Rab11a; palmitoylation; tetraspanin.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Deng Y, Cai S, Shen J, Peng H. Tetraspanins: novel molecular regulators of gastric cancer. Front Oncol. . 2021;11:702510. doi: 10.3389/fonc.2021.702510. - DOI - PMC - PubMed
-
- Stipp CS, Kolesnikova TV, Hemler ME. Functional domains in tetraspanin proteins. Trends Biochem Sci. . 2003;28:106–112. doi: 10.1016/S0968-0004(02)00014-2. - DOI - PubMed
-
- Dodla P, Bhoopalan V, Khoo SK, Miranti C, Sridhar S. Gene expression analysis of human prostate cell lines with and without tumor metastasis suppressor CD82. BMC Cancer. . 2020;20:1211. doi: 10.1186/s12885-020-07675-7. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

