Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy
- PMID: 35538060
- PMCID: PMC9090726
- DOI: 10.1038/s41467-022-30052-w
Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy
Abstract
Safety and effectiveness of COVID-19 vaccines during pregnancy is a particular concern affecting vaccination uptake by this vulnerable group. Here we evaluated evidence from 23 studies including 117,552 COVID-19 vaccinated pregnant people, almost exclusively with mRNA vaccines. We show that the effectiveness of mRNA vaccination against RT-PCR confirmed SARS-CoV-2 infection 7 days after second dose was 89·5% (95% CI 69·0-96·4%, 18,828 vaccinated pregnant people, I2 = 73·9%). The risk of stillbirth was significantly lower in the vaccinated cohort by 15% (pooled OR 0·85; 95% CI 0·73-0·99, 66,067 vaccinated vs. 424,624 unvaccinated, I2 = 93·9%). There was no evidence of a higher risk of adverse outcomes including miscarriage, earlier gestation at birth, placental abruption, pulmonary embolism, postpartum haemorrhage, maternal death, intensive care unit admission, lower birthweight Z-score, or neonatal intensive care unit admission (p > 0.05 for all). COVID-19 mRNA vaccination in pregnancy appears to be safe and is associated with a reduction in stillbirth.
© 2022. The Author(s).
Conflict of interest statement
P.O.B. is co-chair of the Royal College of Obstetricians and Gynaecologists (RCOG) Vaccine Committee. P.O.B. and T.D. are RCOG Vice-Presidents. E.M. is RCOG President. P.H. is a member of the RCOG Vaccine Committee. P.H. is CI of the Preg-Cov trial (UK multicentre COVID vaccination in pregnancy). A.K. is obstetric PI of the Preg-Cov trial (UK multicentre COVID vaccination in pregnancy). A.K. is a member of the COVAX Working Group on COVID vaccination in pregnancy. A.K. is PI of the Pfizer COVID vaccination in pregnancy trial. The remaining authors declare no competing interests.
Figures
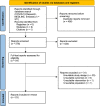

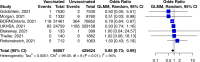
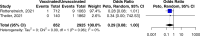

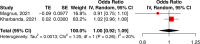
References
-
- ACOG. COVID-19 Vaccination Considerations for Obstetric–gynecologic Care, December 2020 (ACOG, accessed 25 August 2021); https://www.acog.org/clinical/clinical-guidance/practice-advisory/articl....
-
- PHE. JCVI Issues New Advice on COVID-19 Vaccination for Pregnant Women, 2021 [PHE, accessed 25 August 2021); https://www.gov.uk/government/news/jcvi-issues-new-advice-on-covid-19-va....
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

