Serum metabolome associated with severity of acute traumatic brain injury
- PMID: 35538079
- PMCID: PMC9090763
- DOI: 10.1038/s41467-022-30227-5
Serum metabolome associated with severity of acute traumatic brain injury
Abstract
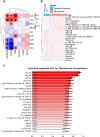
Complex metabolic disruption is a crucial aspect of the pathophysiology of traumatic brain injury (TBI). Associations between this and systemic metabolism and their potential prognostic value are poorly understood. Here, we aimed to describe the serum metabolome (including lipidome) associated with acute TBI within 24 h post-injury, and its relationship to severity of injury and patient outcome. We performed a comprehensive metabolomics study in a cohort of 716 patients with TBI and non-TBI reference patients (orthopedic, internal medicine, and other neurological patients) from the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) cohort. We identified panels of metabolites specifically associated with TBI severity and patient outcomes. Choline phospholipids (lysophosphatidylcholines, ether phosphatidylcholines and sphingomyelins) were inversely associated with TBI severity and were among the strongest predictors of TBI patient outcomes, which was further confirmed in a separate validation dataset of 558 patients. The observed metabolic patterns may reflect different pathophysiological mechanisms, including protective changes of systemic lipid metabolism aiming to maintain lipid homeostasis in the brain.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures