A prospective trial of treatment de-escalation following neoadjuvant paclitaxel/trastuzumab/pertuzumab in HER2-positive breast cancer
- PMID: 35538105
- PMCID: PMC9091255
- DOI: 10.1038/s41523-022-00429-7
A prospective trial of treatment de-escalation following neoadjuvant paclitaxel/trastuzumab/pertuzumab in HER2-positive breast cancer
Abstract
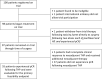
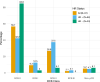
De-escalating adjuvant therapy following pathologic complete response (pCR) to an abbreviated neoadjuvant regimen in human epidermal growth factor receptor 2-positive (HER2+) breast cancer is the focus of international research efforts. However, the feasibility of this approach and its appeal to patients and providers had not been formally investigated. We aimed to assess adherence to de-escalated adjuvant antibody doublet therapy (trastuzumab and pertuzumab [HP], without chemotherapy) among patients with pCR following neoadjuvant paclitaxel/HP (THP). In this single-arm prospective trial, patients with treatment-naïve stage II-III HER2+ breast cancer received neoadjuvant weekly paclitaxel ×12 and HP every 3 weeks ×4. The primary endpoint was receipt of adjuvant non-HER2-directed cytotoxic chemotherapy. Ninety-eight patients received ≥1 dose of THP on study. Patients had median age of 50 years, 86% had stage II tumors, and 34% were hormone receptor-negative. Five patients had incomplete clinical response following THP and received doxorubicin and cyclophosphamide before surgery; they were classified as non-pCR and censored from further analyses. The overall pCR rate was 56.7%. Among patients with pCR, the adherence rate to de-escalated antibody-only therapy (HP) was 98.2% (95% CI 90.3-100.0%), and the primary feasibility endpoint was reached. The majority of patients felt positive or neutral about their adjuvant treatment plans. With brief follow-up (median 19.1 months), there were no breast cancer recurrences. De-escalation of adjuvant chemotherapy among patients who experience pCR in early-stage HER2+ breast cancer is a practicable approach for both patients and physicians. Planned and ongoing prospective trials will determine the long-term efficacy of this approach.Trial registration clinicaltrials.gov, NCT03716180, https://clinicaltrials.gov/ct2/show/NCT03716180 .
© 2022. The Author(s).
Conflict of interest statement
A.G.W.: institutional research support from Genentech, MacroGenics, and Merck. A.H.P.: travel support from Novartis. LMS declares consulting fees from Novartis. O.M.: receives institutional research funding from Abbvie, Genentech/Pfizer, and Roche; honoraria from Roche. S.M.T.: receives institutional research funding from AstraZeneca, Lilly, Merck, Nektar, Novartis, Pfizer, Genentech/Roche, Immunomedics, Exelixis, Bristol-Myers Squibb, Eisai, Nanostring, Sanofi, Cyclacel, Odonate, and Seattle Genetics; has served as an advisor/consultant to AstraZeneca, Lilly, Merck, Nektar, Novartis, Pfizer, Genentech/Roche, Immunomedics, Bristol-Myers Squibb, Eisai, Nanostring, Puma, Sanofi, Celldex, Paxman, Silverback Therapeutics, G1 Therapeutics, Gilead, AbbVie, Anthenex, OncoPep, Outcomes4Me, Kyowa Kirin Pharmaceuticals, Daiichi-Sankyo, Ellipsis, and Samsung Bioepsis Inc. T.A.K.: speakers honoraria Exact Sciences (formerly Genomic Health); faculty, PrecisCa cancer information services and compensated service for a Global Advisory Board of Besins Healthcare. E.A.M.: institutional research from Genentech/Roche via a SU2C grant; research funding from Exact Sciences and Glaxo SmithKline; has served as an advisor/consultant to AstraZeneca, Bristol-Myers Squibb, Exact Sciences, Genentech/Roche, Lilly, Merck and Sellas. E.P.W.: institutional research funding from Genentech/Roche; consultant for Athenex, Carrick Therapeutics, G1 Therapeutics, Genentech/Roche, Genomic Health, Gilead, GSK, Jounce, Lilly, Novartis, Seattle Genetics, Syros, and Zymeworks; scientific advisory board member at Leap Therapeutics; and serves as President-Elect of the American Society of Clinical Oncology (ASCO). All remaining authors have declared no conflicts of interest.
Figures
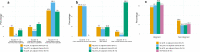
References
-
- Gradishar, W. J. et al. NCCN Clinical Practice Guidelines in Oncology - Breast Cancer, Version 2.2017 (National Comprehensive Cancer Network, 2017).
-
- Gianni L, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17:791–800. doi: 10.1016/S1470-2045(16)00163-7. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

