An updated view into the cell cycle kinetics of human T lymphocytes and the impact of irradiation
- PMID: 35538107
- PMCID: PMC9090834
- DOI: 10.1038/s41598-022-11364-9
An updated view into the cell cycle kinetics of human T lymphocytes and the impact of irradiation
Abstract
Even though a detailed understanding of the proliferative characteristics of T lymphocytes is imperative in many research fields, prior studies have never reached a consensus on these characteristics, and on the corresponding cell cycle kinetics specifically. In this study, the general proliferative response of human T lymphocytes to phytohaemagglutinin (PHA) stimulation was characterized using a carboxyfluorescein succinimidyl ester-based flow cytometric assay. We were able to determine when PHA-stimulated T lymphocytes complete their first division, the proportion of cells that initiate proliferation, the subsequent division rate of the cells, and the impact of irradiation on these proliferative properties. Next, we accurately visualized the cell cycle progression of dividing T lymphocytes cultured in whole blood using an adapted 5-ethynyl-2'-deoxyuridine pulse-chase method. Furthermore, through multiple downstream analysis methods, we were able to make an estimation of the corresponding cell cycle kinetics. We also visualized the impact of X-rays on the progression of the cells through the cell cycle. Our results showed dose-dependent G2 arrest after exposure to irradiation, and a corresponding delay in G1 phase-entry of the cells. In conclusion, utilizing various flow cytometric assays, we provided valuable information on T lymphocyte proliferation characteristics starting from first division to fully dividing cells.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
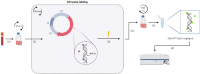
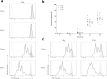

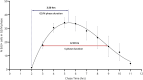
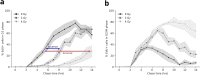
Similar articles
-
Effects of a prospective antitumor agent, 1,4-bis(2'-chloroethyl)-1,4-diazabicyclo-[2.2.1] heptane diperchlorate, on cultured mammalian cells.Cancer Invest. 1984;2(1):1-13. doi: 10.3109/07357908409020281. Cancer Invest. 1984. PMID: 6584191
-
Analysis of Gene Expression Changes in PHA-M Stimulated Lymphocytes - Unraveling PHA Activity as Prerequisite for Dicentric Chromosome Analysis.Radiat Res. 2018 Jun;189(6):579-596. doi: 10.1667/RR14974.1. Epub 2018 Apr 3. Radiat Res. 2018. PMID: 29613823
-
Carboxyfluorescein succinimidyl ester-based proliferative assays for assessment of T cell function in the diagnostic laboratory.Immunol Cell Biol. 1999 Dec;77(6):559-64. doi: 10.1046/j.1440-1711.1999.00870.x. Immunol Cell Biol. 1999. PMID: 10571678
-
Changes in activation markers and cell membrane receptors on human peripheral blood T lymphocytes during cell cycle progression after PHA stimulation.Immunology. 1988 Jul;64(3):419-25. Immunology. 1988. PMID: 3261709 Free PMC article.
-
Methods to Assess Proliferation of Stimulated Human Lymphocytes In Vitro: A Narrative Review.Cells. 2023 Jan 20;12(3):386. doi: 10.3390/cells12030386. Cells. 2023. PMID: 36766728 Free PMC article. Review.
Cited by
-
Designing a linear accelerator-based "X irradiation system" for platelet products: an efficient, safe, accessible and cost-effective alternative for conventional X- or gamma irradiators.Sci Rep. 2024 Nov 17;14(1):28363. doi: 10.1038/s41598-024-80118-6. Sci Rep. 2024. PMID: 39550453 Free PMC article.
-
Replication stress, microcephalic primordial dwarfism, and compromised immunity in ATRIP deficient patients.J Exp Med. 2025 May 5;222(5):e20241432. doi: 10.1084/jem.20241432. Epub 2025 Mar 3. J Exp Med. 2025. PMID: 40029331
-
Osteoimmune Properties of Mesoporous Bioactive Nanospheres: A Study on T Helper Lymphocytes.Nanomaterials (Basel). 2023 Jul 26;13(15):2183. doi: 10.3390/nano13152183. Nanomaterials (Basel). 2023. PMID: 37570501 Free PMC article.
-
Methods for Inferring Cell Cycle Parameters Using Thymidine Analogues.Biology (Basel). 2023 Jun 20;12(6):885. doi: 10.3390/biology12060885. Biology (Basel). 2023. PMID: 37372169 Free PMC article. Review.
-
Neoadjuvant radiotherapy in ER+, HER2+, and triple-negative -specific breast cancer based humanized tumor mice enhances anti-PD-L1 treatment efficacy.Front Immunol. 2024 Apr 29;15:1355130. doi: 10.3389/fimmu.2024.1355130. eCollection 2024. Front Immunol. 2024. PMID: 38742103 Free PMC article.
References
-
- Bernheim JL, Dorian RE, Mendelsohn J. DNA synthesis and proliferation of human lymphocytes in vitro: I. Cell kinetics of response to phytohemagglutinin. J. Immunol. 1978;120:955–962. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

