A non-canonical Raf function is required for dorsal-ventral patterning during Drosophila embryogenesis
- PMID: 35538124
- PMCID: PMC9090920
- DOI: 10.1038/s41598-022-11699-3
A non-canonical Raf function is required for dorsal-ventral patterning during Drosophila embryogenesis
Abstract
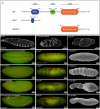
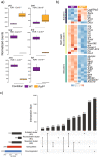
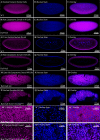
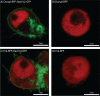
Proper embryonic development requires directional axes to pattern cells into embryonic structures. In Drosophila, spatially discrete expression of transcription factors determines the anterior to posterior organization of the early embryo, while the Toll and TGFβ signalling pathways determine the early dorsal to ventral pattern. Embryonic MAPK/ERK signaling contributes to both anterior to posterior patterning in the terminal regions and to dorsal to ventral patterning during oogenesis and embryonic stages. Here we describe a novel loss of function mutation in the Raf kinase gene, which leads to loss of ventral cell fates as seen through the loss of the ventral furrow, the absence of Dorsal/NFκB nuclear localization, the absence of mesoderm determinants Twist and Snail, and the expansion of TGFβ. Gene expression analysis showed cells adopting ectodermal fates much like loss of Toll signaling. Our results combine novel mutants, live imaging, optogenetics and transcriptomics to establish a novel role for Raf, that appears to be independent of the MAPK cascade, in embryonic patterning.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Signaling between somatic follicle cells and the germline patterns the egg and embryo of Drosophila.Curr Top Dev Biol. 2020;140:55-86. doi: 10.1016/bs.ctdb.2019.10.004. Epub 2019 Nov 19. Curr Top Dev Biol. 2020. PMID: 32591083 Review.
-
The Mirror transcription factor links signalling pathways in Drosophila oogenesis.Dev Genes Evol. 2000 Sep;210(8-9):449-57. doi: 10.1007/s004270000081. Dev Genes Evol. 2000. PMID: 11180850
-
The role of brinker in eggshell patterning.Mech Dev. 2006 May;123(5):395-406. doi: 10.1016/j.mod.2006.03.007. Epub 2006 May 16. Mech Dev. 2006. PMID: 16707253
-
Quantitative Single-Embryo Profile of Drosophila Genome Activation and the Dorsal-Ventral Patterning Network.Genetics. 2016 Apr;202(4):1575-84. doi: 10.1534/genetics.116.186783. Epub 2016 Feb 19. Genetics. 2016. PMID: 26896327 Free PMC article.
-
Maternal AP determinants in the Drosophila oocyte and embryo.Wiley Interdiscip Rev Dev Biol. 2016 Sep;5(5):562-81. doi: 10.1002/wdev.235. Epub 2016 Jun 2. Wiley Interdiscip Rev Dev Biol. 2016. PMID: 27253156 Review.
Cited by
-
Mechanisms for controlling Dorsal nuclear levels.Front Cell Dev Biol. 2024 Aug 5;12:1436369. doi: 10.3389/fcell.2024.1436369. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39161589 Free PMC article. Review.
-
Role of TLRs as signaling cascades to combat infectious diseases: a review.Cell Mol Life Sci. 2025 Mar 19;82(1):122. doi: 10.1007/s00018-025-05631-x. Cell Mol Life Sci. 2025. PMID: 40105962 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

