Radiobiological effects of wound fluid on breast cancer cell lines and human-derived tumor spheroids in 2D and microfluidic culture
- PMID: 35538133
- PMCID: PMC9091274
- DOI: 10.1038/s41598-022-11023-z
Radiobiological effects of wound fluid on breast cancer cell lines and human-derived tumor spheroids in 2D and microfluidic culture
Abstract
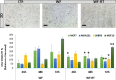
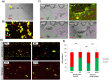
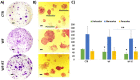
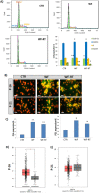
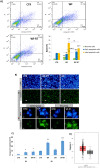
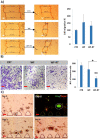
Intraoperative radiotherapy (IORT) could abrogate cancer recurrences, but the underlying mechanisms are unclear. To clarify the effects of IORT-induced wound fluid on tumor progression, we treated breast cancer cell lines and human-derived tumor spheroids in 2D and microfluidic cell culture systems, respectively. The viability, migration, and invasion of the cells under treatment of IORT-induced wound fluid (WF-RT) and the cells under surgery-induced wound fluid (WF) were compared. Our findings showed that cell viability was increased in spheroids under both WF treatments, whereas viability of the cell lines depended on the type of cells and incubation times. Both WFs significantly increased sub-G1 and arrested the cells in G0/G1 phases associated with increased P16 and P21 expression levels. The expression level of Caspase 3 in both cell culture systems and for both WF-treated groups was significantly increased. Furthermore, our results revealed that although the migration was increased in both systems of WF-treated cells compared to cell culture media-treated cells, E-cadherin expression was significantly increased only in the WF-RT group. In conclusion, WF-RT could not effectively inhibit tumor progression in an ex vivo tumor-on-chip model. Moreover, our data suggest that a microfluidic system could be a suitable 3D system to mimic in vivo tumor conditions than 2D cell culture.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Surgical wound fluids from patients treated with intraoperative radiotherapy induce radiobiological response in breast cancer cells.Med Oncol. 2018 Dec 31;36(2):14. doi: 10.1007/s12032-018-1243-z. Med Oncol. 2018. PMID: 30599057 Free PMC article.
-
Wound fluids collected postoperatively from patients with breast cancer induce epithelial to mesenchymal transition but intraoperative radiotherapy impairs this effect by activating the radiation-induced bystander effect.Sci Rep. 2019 May 27;9(1):7891. doi: 10.1038/s41598-019-44412-y. Sci Rep. 2019. PMID: 31133667 Free PMC article.
-
Surgical Wound Fluids from Patients with Breast Cancer Reveal Similarities in the Biological Response Induced by Intraoperative Radiation Therapy and the Radiation-Induced Bystander Effect-Transcriptomic Approach.Int J Mol Sci. 2020 Feb 10;21(3):1159. doi: 10.3390/ijms21031159. Int J Mol Sci. 2020. PMID: 32050557 Free PMC article.
-
Generation of Multicellular Breast Cancer Tumor Spheroids: Comparison of Different Protocols.J Mammary Gland Biol Neoplasia. 2016 Dec;21(3-4):89-98. doi: 10.1007/s10911-016-9359-2. Epub 2016 Aug 12. J Mammary Gland Biol Neoplasia. 2016. PMID: 27518775 Review.
-
Three-dimensional culture models: emerging platforms for screening the antitumoral efficacy of nanomedicines.Nanomedicine (Lond). 2023 Mar;18(7):633-647. doi: 10.2217/nnm-2022-0205. Epub 2023 May 15. Nanomedicine (Lond). 2023. PMID: 37183804 Review.
Cited by
-
Biological and clinical review of IORT-induced wound fluid in breast cancer patients.Front Oncol. 2022 Nov 21;12:980513. doi: 10.3389/fonc.2022.980513. eCollection 2022. Front Oncol. 2022. PMID: 36479071 Free PMC article. Review.
-
Cancer-on-chip: a 3D model for the study of the tumor microenvironment.J Biol Eng. 2023 Aug 17;17(1):53. doi: 10.1186/s13036-023-00372-6. J Biol Eng. 2023. PMID: 37592292 Free PMC article. Review.
-
Bridging the gap: advancing cancer cell culture to reveal key metabolic targets.Front Oncol. 2024 Sep 17;14:1480613. doi: 10.3389/fonc.2024.1480613. eCollection 2024. Front Oncol. 2024. PMID: 39355125 Free PMC article. Review.
References
-
- Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: A systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749–1768. doi: 10.1001/jamaoncol.2019.2996. - DOI - PMC - PubMed
-
- Moaiery H, Akbari ME, Nafissi N, Mahdavi SR, Reza H. Intraoperative electron radiotherapy (IOERT) boost versus external beam radiotherapy (EBRT) boost in invasive lobular carcinoma breast cancer cases. Int. J. Cancer Manag. 2018;11(7):e69364. doi: 10.5812/ijcm.69364. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

