Short treatment of peripheral blood cells product with Fas ligand using closed automated cell processing system significantly reduces immune cell reactivity of the graft in vitro and in vivo
- PMID: 35538142
- PMCID: PMC9088133
- DOI: 10.1038/s41409-022-01698-3
Short treatment of peripheral blood cells product with Fas ligand using closed automated cell processing system significantly reduces immune cell reactivity of the graft in vitro and in vivo
Abstract
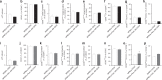
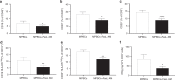
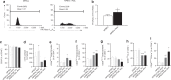
Mobilized peripheral blood cells (MPBCs) graft and peripheral blood cells apheresis are used for bone marrow transplantation and for treatment of graft versus host disease (GvHD). We demonstrate that a short treatment of MPBCs with Fas ligand (FasL, CD95L) for 2 h using a closed automated cell processing system selectively induces apoptosis of specific donor T cells, B cells and antigen presenting cells, but, critically, not CD34+ hematopoietic stem cells and progenitors, all of which may contribute to an increased likelihood of graft survival and functionality and reduced GvHD. Treated cells secreted lower levels of interferon-gamma as compared with control, untreated, cells. Moreover, FasL treatment of immune cells increased signals, which led to their phagocytosis by activated macrophages. FasL treated immune cells also reduced the ability of activated macrophages to secrete pro-inflammatory cytokines. Most importantly, FasL ex vivo treated MPBCs prior to transplantation in NOD-SCID NSG mice prevented GvHD and improved stem cell transplantation in vivo. In conclusion, MPBCs, as well as other blood cell products, treated with FasL by automated manufacturing (AM), may be used as potential treatments for conditions where the immune system is over-responding to both self and non-self-antigens.
© 2022. The Author(s).
Conflict of interest statement
AO, GR, MR, MST, EG, and MK are employees of Cellect Biotherapeutics, and SY is an employee and shareholder of the same concern. EZ-K is an employee of Goldyne Savad Institute of Gene Therapy, Hebrew University of Jerusalem, Israel. AP serves as a paid consultant for Cellect Biotherapeutics. This technology is covered by patents and pending patents.
Figures



Similar articles
-
Brief ex vivo Fas-ligand incubation attenuates GvHD without compromising stem cell graft performance.Bone Marrow Transplant. 2020 Jul;55(7):1305-1316. doi: 10.1038/s41409-020-0941-2. Epub 2020 May 20. Bone Marrow Transplant. 2020. PMID: 32433499 Free PMC article.
-
Depletion of naïve lymphocytes with fas ligand ex vivo prevents graft-versus-host disease without impairing T cell support of engraftment or graft-versus-tumor activity.Biol Blood Marrow Transplant. 2013 Feb;19(2):185-95. doi: 10.1016/j.bbmt.2012.10.004. Epub 2012 Oct 16. Biol Blood Marrow Transplant. 2013. PMID: 23078782
-
Apoptotic signaling through Fas and TNF receptors ameliorates GVHD in mobilized peripheral blood grafts.Bone Marrow Transplant. 2014 May;49(5):640-8. doi: 10.1038/bmt.2014.12. Epub 2014 Feb 24. Bone Marrow Transplant. 2014. PMID: 24566711
-
Hematopoietic stem cell graft manipulation as a mechanism of immunotherapy.Int Immunopharmacol. 2003 Aug;3(8):1121-43. doi: 10.1016/S1567-5769(03)00014-6. Int Immunopharmacol. 2003. PMID: 12860168 Review.
-
Protective conditioning against GVHD and graft rejection after combined organ and hematopoietic cell transplantation.Blood Cells Mol Dis. 2008 Jan-Feb;40(1):48-54. doi: 10.1016/j.bcmd.2007.06.019. Epub 2007 Sep 10. Blood Cells Mol Dis. 2008. PMID: 17827036 Review.
Cited by
-
Cannabidiol regulates apoptosis and autophagy in inflammation and cancer: A review.Front Pharmacol. 2023 Jan 23;14:1094020. doi: 10.3389/fphar.2023.1094020. eCollection 2023. Front Pharmacol. 2023. PMID: 36755953 Free PMC article. Review.
-
A Novel Scientometrics Research on the Interaction between Oxidative Stress and Hematopoietic Stem Cell Transplantation Complications: From Graft-versus-Host Disease to Sepsis.Oxid Med Cell Longev. 2023 Jan 27;2023:7708085. doi: 10.1155/2023/7708085. eCollection 2023. Oxid Med Cell Longev. 2023. PMID: 36743696 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

