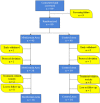
The impact of metformin use on the outcomes of locally advanced breast cancer patients receiving neoadjuvant chemotherapy: an open-labelled randomized controlled trial
- PMID: 35538143
- PMCID: PMC9091204
- DOI: 10.1038/s41598-022-11138-3
The impact of metformin use on the outcomes of locally advanced breast cancer patients receiving neoadjuvant chemotherapy: an open-labelled randomized controlled trial
Abstract
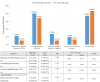
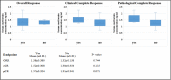
Recently, several clinical trials have attempted to find evidence that supports the anticancer use of metformin in breast cancer (BC) patients. The current study evaluates the anticancer activity of metformin in addition to neoadjuvant chemotherapy (NACT) in locally advanced BC patients. Additionally, we assess the safety and tolerability of this combination and its effect on the quality of life (QoL) of BC patients. Eighty non-diabetic female patients with proven locally advanced BC were randomized into two arms. The first arm received anthracycline/taxane-based NACT plus metformin. The second arm received anthracycline/taxane-based NACT only. Overall response rate (ORR), clinical complete response (cCr), pathological complete response (pCR), and breast conservative rate (BCR) were evaluated between both groups, and correlated with serum metformin concentration. ORR, cCr, pCR, and BCR increased non-significantly in the metformin group compared to the control group; 80.6% vs 68.4%, 27.8% vs 10.5%, 22.2% vs 10.5%, and 19.4% vs 13.2%, respectively. A trend towards cCR and pCR was associated with higher serum metformin concentrations. Metformin decreased the incidence of peripheral neuropathy, bone pain, and arthralgia, although worsened the gastrointestinal adverse events. Metformin combination with NACT has no effect on the QoL of BC patients. Metformin combination with NACT is safe, tolerable, and improves non-significantly the clinical and pathological tumor response of BC patients.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Neoadjuvant chemotherapy in patients with locally advanced breast cancer: A pilot-observational study.J Cancer Res Ther. 2015 Jul-Sep;11(3):612-6. doi: 10.4103/0973-1482.146056. J Cancer Res Ther. 2015. PMID: 26458590
-
Pathological complete response in invasive breast cancer treated by skin sparing mastectomy and immediate reconstruction following neoadjuvant chemotherapy and radiation therapy: Comparison between immunohistochemical subtypes.Breast. 2017 Apr;32:37-43. doi: 10.1016/j.breast.2016.12.014. Epub 2016 Dec 26. Breast. 2017. PMID: 28033508
-
Effects of adding taxane to anthracycline-based neoadjuvant chemotherapy in locally advanced breast cancer.Med Glas (Zenica). 2019 Feb 1;16(1):77-82. doi: 10.17392/964-19. Med Glas (Zenica). 2019. PMID: 30256061 Clinical Trial.
-
Sequencing of anthracyclines and taxanes in neoadjuvant and adjuvant therapy for early breast cancer.Cochrane Database Syst Rev. 2019 Feb 18;2(2):CD012873. doi: 10.1002/14651858.CD012873.pub2. Cochrane Database Syst Rev. 2019. PMID: 30776132 Free PMC article.
-
Neoadjuvant docetaxel in locally advanced breast cancer.Breast Cancer Res Treat. 2003;79 Suppl 1:S19-24. doi: 10.1023/a:1024333725148. Breast Cancer Res Treat. 2003. PMID: 12868802 Review.
Cited by
-
Obesity-specific improvement of lung cancer outcomes and immunotherapy efficacy with metformin.J Natl Cancer Inst. 2025 Apr 1;117(4):673-684. doi: 10.1093/jnci/djae295. J Natl Cancer Inst. 2025. PMID: 39560490
-
Chemotherapy-induced peripheral neuropathy in children and adolescent cancer patients.Front Mol Biosci. 2022 Oct 14;9:1015746. doi: 10.3389/fmolb.2022.1015746. eCollection 2022. Front Mol Biosci. 2022. PMID: 36310587 Free PMC article. Review.
-
Repurposing of Metformin to Improve Survival Outcomes in Patients With Upper Tract Urothelial Carcinoma.Cancer Med. 2025 Jan;14(1):e70567. doi: 10.1002/cam4.70567. Cancer Med. 2025. PMID: 39757744 Free PMC article.
-
Current status and frontier tracking of clinical trials on Metformin for cancer treatment.J Cancer Res Clin Oncol. 2023 Dec;149(18):16931-16946. doi: 10.1007/s00432-023-05391-w. Epub 2023 Sep 12. J Cancer Res Clin Oncol. 2023. PMID: 37698682 Free PMC article. Review.
-
Neoadjuvant chemotherapy with or without metformin in early-stage or locally advanced (non-metastatic) triple negative breast cancer-an open-label phase 2 randomized controlled trial.Front Oncol. 2025 Jun 25;15:1561692. doi: 10.3389/fonc.2025.1561692. eCollection 2025. Front Oncol. 2025. PMID: 40636683 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical