Nuclear soluble cGAS senses double-stranded DNA virus infection
- PMID: 35538147
- PMCID: PMC9090744
- DOI: 10.1038/s42003-022-03400-1
Nuclear soluble cGAS senses double-stranded DNA virus infection
Abstract
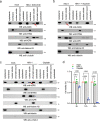
The DNA sensor cGAS detects cytosolic DNA and instigates type I interferon (IFN) expression. Recent studies find that cGAS also localizes in the nucleus and binds the chromatin. Despite the mechanism controlling nuclear cGAS activation is well elucidated, whether nuclear cGAS participates in DNA sensing is unclear. Here, we report that herpes simplex virus 1 (HSV-1) infection caused the release of cGAS from the chromatin into the nuclear soluble fraction. Like its cytosolic counterpart, the leaked nuclear soluble cGAS also sensed viral DNA, produced cGAMP, and induced mRNA expression of type I IFN and interferon-stimulated genes. Consistently, the nuclear soluble cGAS limited HSV-1 infection. Furthermore, enzyme-deficient mutation (D307A) or cGAS inhibitor RU.251 abolished nuclear cGAS-mediated innate immune responses, suggesting that enzymatic activity is also required for nuclear soluble cGAS. Taken all together, our study demonstrates that nuclear soluble cGAS acts as a nuclear DNA sensor detecting nuclear-replicating DNA viruses.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Herpes simplex virus infected cell protein 8 is required for viral inhibition of the cGAS pathway.Virology. 2023 Aug;585:34-41. doi: 10.1016/j.virol.2023.05.002. Epub 2023 May 25. Virology. 2023. PMID: 37271042 Free PMC article.
-
Glutamylation of the DNA sensor cGAS regulates its binding and synthase activity in antiviral immunity.Nat Immunol. 2016 Apr;17(4):369-78. doi: 10.1038/ni.3356. Epub 2016 Feb 1. Nat Immunol. 2016. PMID: 26829768
-
The 4EHP-mediated translational repression of cGAS impedes the host immune response against DNA viruses.Proc Natl Acad Sci U S A. 2024 Nov 26;121(48):e2413018121. doi: 10.1073/pnas.2413018121. Epub 2024 Nov 19. Proc Natl Acad Sci U S A. 2024. PMID: 39560640 Free PMC article.
-
Function and Regulation of Nuclear DNA Sensors During Viral Infection and Tumorigenesis.Front Immunol. 2021 Jan 11;11:624556. doi: 10.3389/fimmu.2020.624556. eCollection 2020. Front Immunol. 2021. PMID: 33505405 Free PMC article. Review.
-
Targeting of the cGAS-STING system by DNA viruses.Biochem Pharmacol. 2020 Apr;174:113831. doi: 10.1016/j.bcp.2020.113831. Epub 2020 Jan 28. Biochem Pharmacol. 2020. PMID: 32004549 Review.
Cited by
-
The role of cGAS in epithelial dysregulation in inflammatory bowel disease and gastrointestinal malignancies.Front Pharmacol. 2024 Jul 10;15:1409683. doi: 10.3389/fphar.2024.1409683. eCollection 2024. Front Pharmacol. 2024. PMID: 39050748 Free PMC article. Review.
-
Centromeres as minefields: host-virus warfare.Cell Res. 2025 Aug 1. doi: 10.1038/s41422-025-01159-8. Online ahead of print. Cell Res. 2025. PMID: 40750685 No abstract available.
-
Catch me if you can: viral nucleic acids to host sensors.Front Immunol. 2025 Jul 28;16:1632283. doi: 10.3389/fimmu.2025.1632283. eCollection 2025. Front Immunol. 2025. PMID: 40791597 Free PMC article. Review.
-
Airway epithelial cGAS inhibits LPS-induced acute lung injury through CREB signaling.Cell Death Dis. 2023 Dec 19;14(12):844. doi: 10.1038/s41419-023-06364-0. Cell Death Dis. 2023. PMID: 38114479 Free PMC article.
-
cGAS: action in the nucleus.Front Immunol. 2024 Mar 7;15:1380517. doi: 10.3389/fimmu.2024.1380517. eCollection 2024. Front Immunol. 2024. PMID: 38515746 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

