Investigating the presence of adsorbed species on Pt steps at low potentials
- PMID: 35538173
- PMCID: PMC9090771
- DOI: 10.1038/s41467-022-30241-7
Investigating the presence of adsorbed species on Pt steps at low potentials
Erratum in
-
Author Correction: Investigating the presence of adsorbed species on Pt steps at low potentials.Nat Commun. 2022 Jun 28;13(1):3705. doi: 10.1038/s41467-022-31404-2. Nat Commun. 2022. PMID: 35764650 Free PMC article. No abstract available.
Abstract
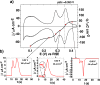
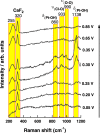
The study of the OH adsorption process on Pt single crystals is of paramount importance since this adsorbed species is considered the main intermediate in many electrochemical reactions of interest, in particular, those oxidation reactions that require a source of oxygen. So far, it is frequently assumed that the OH adsorption on Pt only takes place at potentials higher than 0.55 V (versus the reversible hydrogen electrode), regardless of the Pt surface structure. However, by CO displacement experiments, alternating current voltammetry, and Raman spectroscopy, we demonstrate here that OH is adsorbed at more negative potentials on the low coordinated Pt atoms, the Pt steps. This finding opens a new door in the mechanistic study of many relevant electrochemical reactions, leading to a better understanding that, ultimately, can be essential to reach the final goal of obtaining improved catalysts for electrochemical applications of technological interest.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Rizo R, Arán-Ais RM, Herrero E. On the oxidation mechanism of C1-C2 organic molecules on platinum. A comparative analysis. Curr. Opin. Electrochem. 2021;25:100648. doi: 10.1016/j.coelec.2020.100648. - DOI
-
- Korzeniewski, C., Climent, V. & Feliu, J. Electrochemistry at Platinum Single Crystal Electrodes. in Electroanalytical Chemistry A Series of Advances: Volume 24 (eds. Bard, A. J. & Zoski, C. G.) 75–170 (CRC Press, 2011).
-
- McCrum IT, Koper MTM. The role of adsorbed hydroxide in hydrogen evolution reaction kinetics on modified platinum. Nat. Energy. 2020;5:891–899. doi: 10.1038/s41560-020-00710-8. - DOI
-
- Rizo R, Feliu JM, Herrero E. New insights into the hydrogen peroxide reduction reaction and its comparison with the oxygen reduction reaction in alkaline media on well-defined platinum surfaces. J. Catal. 2021;398:123–132. doi: 10.1016/j.jcat.2021.04.018. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

