Quorum quenching of Streptococcus mutans via the nano-quercetin-based antimicrobial photodynamic therapy as a potential target for cariogenic biofilm
- PMID: 35538403
- PMCID: PMC9088123
- DOI: 10.1186/s12866-022-02544-8
Quorum quenching of Streptococcus mutans via the nano-quercetin-based antimicrobial photodynamic therapy as a potential target for cariogenic biofilm
Abstract
Background: Quorum sensing (QS) system can regulate the expression of virulence factors and biofilm formation in Streptococcus mutans. Antimicrobial photodynamic therapy (aPDT) inhibits quorum quenching (QQ), and can be used to prevent microbial biofilm. We thereby aimed to evaluate the anti-biofilm potency and anti-metabolic activity of nano-quercetin (N-QCT)-mediated aPDT against S. mutans. Also, in silico evaluation of the inhibitory effect of N-QCT on the competence-stimulating peptide (CSP) of S. mutans was performed to elucidate the impact of aPDT on various QS-regulated genes.
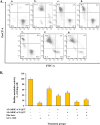
Methods: Cytotoxicity and intracellular reactive oxygen species (ROS) generation were assessed following synthesis and confirmation of N-QCT. Subsequently, the minimum biofilm inhibitory concentration (MBIC) of N-QCT against S. mutans and anti-biofilm effects of aPDT were assessed using colorimetric assay and plate counting. Molecular modeling and docking analysis were performed to confirm the connection of QCT to CSP. The metabolic activity of S. mutans and the expression level of various genes involved in QS were evaluated by flow cytometry and reverse transcription quantitative real-time PCR, respectively.
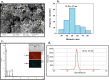
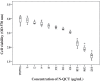
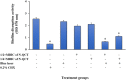
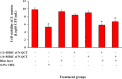
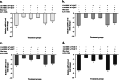
Results: Successful synthesis of non-toxic N-QCT was confirmed through several characterization tests. The MBIC value of N-QCT against S. mutans was 128 μg/mL. Similar to the crystal violet staining, the results log10 CFU/mL showed a significant degradation of preformed biofilms in the group treated with aPDT compared to the control group (P < 0.05). Following aPDT, metabolic activity of S. mutans also decreased by 85.7% (1/2 × MBIC of N-QCT) and 77.3% (1/4 × MBIC of N-QCT), as compared to the control values (P < 0.05). In silico analysis showed that the QCT molecule was located in the site formed by polypeptide helices of CSP. The relative expression levels of the virulence genes were significantly decreased in the presence of N-QCT-mediated aPDT (P < 0.05).
Conclusions: The combination of N-QCT with blue laser as a QQ-strategy leads to maximum ROS generation, disrupts the microbial biofilm of S. mutans, reduces metabolic activity, and downregulates the expression of genes involved in the QS pathway by targeting genes of the QS signaling system of S. mutans.
Keywords: Antimicrobial photodynamic therapy; Competence-stimulating peptide; Dental caries; Quorum sensing; Streptococcus mutans.
© 2022. The Author(s).
Conflict of interest statement
There is no competing interest.
Figures





Similar articles
-
Theranostic nanoplatforms of emodin-chitosan with blue laser light on enhancing the anti-biofilm activity of photodynamic therapy against Streptococcus mutans biofilms on the enamel surface.BMC Microbiol. 2022 Mar 4;22(1):68. doi: 10.1186/s12866-022-02481-6. BMC Microbiol. 2022. PMID: 35246026 Free PMC article.
-
Anti-biofilm and anti-metabolic effects of antimicrobial photodynamic therapy using chlorophyllin-phycocyanin mixture against Streptococcus mutans in experimental biofilm caries model on enamel slabs.Photodiagnosis Photodyn Ther. 2020 Mar;29:101620. doi: 10.1016/j.pdpdt.2019.101620. Epub 2019 Dec 10. Photodiagnosis Photodyn Ther. 2020. PMID: 31841686
-
Ex-vivo effects of propolis quantum dots-nisin-nanoquercetin-mediated photodynamic therapy on Streptococcus mutans biofilms and white spot lesions.Photodiagnosis Photodyn Ther. 2023 Mar;41:103255. doi: 10.1016/j.pdpdt.2022.103255. Epub 2022 Dec 22. Photodiagnosis Photodyn Ther. 2023. PMID: 36567010
-
Quorum sensing and biofilm formation by Streptococcus mutans.Adv Exp Med Biol. 2008;631:178-88. doi: 10.1007/978-0-387-78885-2_12. Adv Exp Med Biol. 2008. PMID: 18792689 Review.
-
Effect of bioactive compounds on the regulation of quorum sensing network-associated genes and virulence in Streptococcus mutans-A systematic review.Arch Oral Biol. 2020 Nov;119:104893. doi: 10.1016/j.archoralbio.2020.104893. Epub 2020 Aug 31. Arch Oral Biol. 2020. PMID: 32961379
Cited by
-
Enhancing antimicrobial efficacy against Pseudomonas aeruginosa biofilm through carbon dot-mediated photodynamic inactivation.AMB Express. 2024 Sep 28;14(1):108. doi: 10.1186/s13568-024-01766-5. AMB Express. 2024. PMID: 39342036 Free PMC article.
-
Combatting biofilm-mediated infections in clinical settings by targeting quorum sensing.Cell Surf. 2024 Nov 8;12:100133. doi: 10.1016/j.tcsw.2024.100133. eCollection 2024 Dec. Cell Surf. 2024. PMID: 39634722 Free PMC article. Review.
-
Polyphenolic natural products as photosensitizers for antimicrobial photodynamic therapy: recent advances and future prospects.Front Immunol. 2023 Oct 31;14:1275859. doi: 10.3389/fimmu.2023.1275859. eCollection 2023. Front Immunol. 2023. PMID: 38022517 Free PMC article. Review.
-
Postbiotic mediators derived from Lactobacillus species enhance riboflavin-mediated antimicrobial photodynamic therapy for eradication of Streptococcus mutans planktonic and biofilm growth.BMC Oral Health. 2024 Jul 24;24(1):836. doi: 10.1186/s12903-024-04620-z. BMC Oral Health. 2024. PMID: 39048998 Free PMC article.
-
Anti-virulence effect of photoactivated nano-quercetin by diode laser on Aggregatibacter actinomycetemcomitans.AMB Express. 2025 Mar 28;15(1):55. doi: 10.1186/s13568-025-01868-8. AMB Express. 2025. PMID: 40153184 Free PMC article.
References
-
- Medapati MR, Singh N, Bhagirath AY, Duan K, Triggs-Raine B, Batista EL, Jr, Chelikani P. Bitter taste receptor T2R14 detects quorum sensing molecules from cariogenic Streptococcus mutans and mediates innate immune responses in gingival epithelial cells. FASEB J. 2021;35(3):e21375. doi: 10.1096/fj.202000208R. - DOI - PubMed
-
- Bikash CR, Tal-Gan Y. Identification of highly potent competence stimulating peptide-based quorum sensing activators in Streptococcus mutans through the utilization of N-methyl and reverse alanine scanning. Bioorg Med Chem Lett. 2019;29(6):811–814. doi: 10.1016/j.bmcl.2019.01.029. - DOI - PMC - PubMed
-
- Bikash CR, Hamry SR, Tal-Gan Y. Structure–activity relationships of the competence stimulating peptide in Streptococcus mutans reveal motifs critical for membrane protease SepM recognition and ComD receptor activation. ACS Infect Dis. 2018;4(9):1385–1394. doi: 10.1021/acsinfecdis.8b00115. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

