Neuroendocrine differentiation distinguishes basaloid variant of lung squamous cell carcinoma
- PMID: 35538551
- PMCID: PMC9088121
- DOI: 10.1186/s13000-022-01223-6
Neuroendocrine differentiation distinguishes basaloid variant of lung squamous cell carcinoma
Abstract
Background: Neuroendocrine (NE) differentiation is widely studied in non-small cell lung carcinomas (NSCLC) however, its significance remains unclear in basaloid squamous cell carcinomas (B-SqCC). This study aims to assess the extent of NE differentiation in B-SqCC and characterize the underlying molecular process.
Methods: This study evaluated resected B-SqCC, small cell lung cancer (SCLC) and poorly differentiated SqCC (PD-SqCC) from 2005 to 2020 at the Ottawa Hospital. Samples were subject to pathological review, immunohistochemistry (IHC) and survival analysis. Gene expression analysis was performed on B-SqCC samples exhibiting NE+ and NE- regions (paired samples) to identify differentially expressed genes (DEGs). These DEGs were subsequently validated in unpaired B-SqCC and TCGA samples.
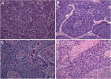
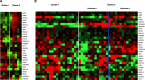
Results: B-SqCC cases were more likely to exhibit nuclear molding, resetting and peripheral palisading than PD-SqCC. B-SqCC were also more likely to demonstrate NE differentiation compared to PD-SqCC (p = 0.006). Pure basaloid squamous cell carcinoma (PB-SqCC) experienced poorer disease-free survival (HR = 3.12, p = 0.043) adjusted for stage. Molecular characterization of paired B-SqCC samples demonstrated DEGs implicated in NOTCH signaling, SCLC and pulmonary neuroendocrine differentiation. Hierarchical clustering using discovered DEGs in unpaired B-SqCC samples distinguished tumors based on NE status (p = 0.048). Likewise, clustering The Cancer Genome Atlas (TCGA) samples with DEGs distinguished B-SqCC from SqCC samples (p = 0.0094).
Conclusion: This study provides IHC and molecular evidence of significant NE-differentiation in B-SqCC and demonstrates their aggressive clinical behavior. These findings suggest that B-SqCC are biologically distinct from SqCC and share characteristics with SCLC.
Keywords: Basaloid squamous cell carcinoma; Gene expression; Immunohistochemistry; NOTCH; Neuroendocrine differentiation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests in this section.
Figures


Similar articles
-
CD56 expression in basaloid anal squamous cell carcinoma - A potential diagnostic pitfall.Ann Diagn Pathol. 2021 Aug;53:151758. doi: 10.1016/j.anndiagpath.2021.151758. Epub 2021 May 8. Ann Diagn Pathol. 2021. PMID: 33989959
-
Prognostic implications of neuroendocrine differentiation and hormone production in patients with Stage I nonsmall cell lung carcinoma.Cancer. 2003 May 15;97(10):2487-97. doi: 10.1002/cncr.11376. Cancer. 2003. PMID: 12733148
-
ΔNp63 (p40) distribution inside lung cancer: a driver biomarker approach to tumor characterization.Int J Surg Pathol. 2013 Jun;21(3):229-39. doi: 10.1177/1066896913476750. Epub 2013 Mar 12. Int J Surg Pathol. 2013. PMID: 23486764
-
Clinicopathological significance of peritumoral alveolar macrophages in patients with resected early-stage lung squamous cell carcinoma.Cancer Immunol Immunother. 2023 Jul;72(7):2205-2215. doi: 10.1007/s00262-023-03393-8. Epub 2023 Mar 2. Cancer Immunol Immunother. 2023. PMID: 36862151 Free PMC article. Review.
-
[Squamous cell carcinoma, basaloid squamous cell carcinoma and adenosquamous carcinoma in the lung].Ann Pathol. 2016 Jan;36(1):15-23. doi: 10.1016/j.annpat.2015.11.011. Epub 2015 Dec 30. Ann Pathol. 2016. PMID: 26746368 Review. French.
Cited by
-
Survival comparison of pulmonary neuroendocrine carcinoma, adenocarcinoma with neuroendocrine differentiation, and adenocarcinoma.J Thorac Dis. 2024 Jan 30;16(1):604-614. doi: 10.21037/jtd-23-1811. Epub 2024 Jan 17. J Thorac Dis. 2024. PMID: 38410570 Free PMC article.
-
POU2F3 in SCLC: Clinicopathologic and Genomic Analysis With a Focus on Its Diagnostic Utility in Neuroendocrine-Low SCLC.J Thorac Oncol. 2022 Sep;17(9):1109-1121. doi: 10.1016/j.jtho.2022.06.004. Epub 2022 Jun 24. J Thorac Oncol. 2022. PMID: 35760287 Free PMC article.
-
Comparison of CT findings between basaloid squamous cell carcinoma and non-basaloid squamous cell carcinoma of the lung.Sci Rep. 2024 Dec 28;14(1):31371. doi: 10.1038/s41598-024-82896-5. Sci Rep. 2024. PMID: 39732972 Free PMC article.
References
-
- Warth A, Botling J, Chung J, Ishii F, Rossi G, Wistuba I. Squamous cell carcinoma of the lung. In: WHO Classification of Tumours Editorial Board. Thoracic tumours. Lyon (France): International Agency for Research on Cancer; 2021. (WHO classification of tumours series, 5th ed.; vol. 5). Available from: https://publications.iarc.fr/595.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

