Patient-derived xenograft (PDX) models, applications and challenges in cancer research
- PMID: 35538576
- PMCID: PMC9088152
- DOI: 10.1186/s12967-022-03405-8
Patient-derived xenograft (PDX) models, applications and challenges in cancer research
Abstract
The establishing of the first cancer models created a new perspective on the identification and evaluation of new anti-cancer therapies in preclinical studies. Patient-derived xenograft models are created by tumor tissue engraftment. These models accurately represent the biology and heterogeneity of different cancers and recapitulate tumor microenvironment. These features have made it a reliable model along with the development of humanized models. Therefore, they are used in many studies, such as the development of anti-cancer drugs, co-clinical trials, personalized medicine, immunotherapy, and PDX biobanks. This review summarizes patient-derived xenograft models development procedures, drug development applications in various cancers, challenges and limitations.
Keywords: Avatar model of cancer; Cancer animal model; Humanized model; PDX; Preclinical study.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

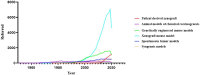

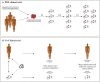
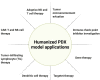
References
-
- Pott P. Chirurgical observations relative to the cataract, the polypus of the nose, the cancer of the scrotum, the different kinds of ruptures, and the mortification of the toes and feet, by Percivall Pott, FRS Surgeon to St. Bartholomew’s-Hospital. Printed, by TJ Carnegy, For L. Hawes, W. Clarke, and R. Collins, in Pater-noster Row; 1775.
-
- Hedrich HJ. The house mouse as a laboratory model: a historical perspective. The Laboratory Mouse. Burlington: Elsevier Science; 2012. p. 868.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

